The role of antipsychotic maintenance medication in symptom control and the prevention of relapse in schizophrenia is well established. However, the success of antipsychotic treatment is reduced by patients’ partial or total non-adherence to medication regimens (Reference Lindstrom and BingeforsLindstrom & Bingefors, 2000). Non-adherence in schizophrenia is a major, preventable cause of morbidity, with significant personal, social and economic costs. Long-acting depot antipsychotics were developed in the 1960s and were specifically aimed at promoting treatment adherence (compliance) in people with chronic illness, thereby enhancing relapse prevention (Reference Davis, Matalon and WatanabeDavis et al, 1994; Reference Weiden and GlazerWeiden & Glazer, 1997). Depot antipsychotics generally consist of an ester of the antipsychotic drug injected intramuscularly in an oily solution every 1–6 weeks (Reference Davis, Matalon and WatanabeDavis et al, 1994). Here we examine the reasons why long-acting antipsychotics are currently underutilised for maintenance treatment and highlight why they are potentially beneficial to patients with schizophrenia. We also consider the future role of depot antipsychotics in the psychiatrist’s armamentarium.
Reasons for non-adherence
The reasons for non-adherence are complex but include such factors as beliefs about the illness and medication. The theoretical framework of the ‘health belief model’ indicates that health behaviour is determined by beliefs that fall into four main categories: benefits, costs, susceptibility to relapse and secondary benefits of medication and treatment adherence (Reference Hughes, Hill and BuddHughes et al, 1997). Even within a relatively compliant group of patients, widely varying degrees of insight and a large range of health beliefs will exist, which, in turn, are important contributors to treatment adherence. Reference Smith, Hughes and BuddSmith et al(1999) suggest that non-adherence is significantly associated with patients’ perception that they have a low susceptibility to relapse (a component of poor insight) and that this is therefore more important than their perceptions and beliefs about potential side-effects.
For patients with schizophrenia, Reference Cramer and RosenheckCramer & Rosenheck (1998) reported an average adherence rate for all antipsychotics of 58% (range 24–90%). Two reviews on depot medication suggest a non-adherence rate of 24% (range 0–54%) (Reference Young, Zonana and SheplerYoung et al, 1986, Reference Young, Spitz and Hillbrand1999). Reference Hogan, Awad and EastwoodHogan et al(1983) demonstrated that patients’ experience of and adherence to antipsychotic regimens depended on how they felt on medication, rather than what they knew or believed about it. Reference AwadAwad (1993) found a correlation between altered subjective state on antipsychotics and medication adherence. Factors implicated in the genesis of such altered states included the patients’ values and attitudes towards health and illness, as well as demographics, psychiatric characteristics, type and dose of antipsychotic, depressive states and extrapyramidal symptoms (Reference GerlachGerlach, 1995; Reference Patel and DavidPatel & David, 2004). In other words, people who are not well disposed towards a particular treatment may be more alert to possible side-effects and more inclined to report them. Reference Weiden, Rapkin and MottWeiden et al(1994) suggested, however, that patients’ attitudes to medication may be completely different from their actual medication-taking behaviour.
Improving treatment adherence has rightly become an important area of clinical research (Reference Lindstrom and BingeforsLindstrom & Bingefors, 2000; Reference Zygmunt, Olfson and BoyerZygmunt et al, 2002; Reference Patel and DavidPatel & David, 2004). In general, simple psychoeducative methods in isolation are not effective, whereas cognitive–behavioural techniques are more likely to succeed (Reference Kemp, Kirov and EverittKemp et al, 1998). Reference KisslingKissling (1994, Reference Kissling1997) suggested that depot antipsychotics should be considered for relapse prevention but that it is neither ethically nor economically defensible to perform relapse prevention of schizophrenic psychosis without measures aimed at improving treatment adherence. However, dedicated time and resources for this remain rare.
The role of long-acting depot antipsychotics
With the relatively recent introduction of the oral atypical antipsychotics, there has been a shift away from depot typicals. The main driving force for this was the reduction in side-effects and the presumed lowered risk of tardive dyskinesia with the atypicals. Nevertheless, until the guidelines on antipsychotic prescribing were released by the National Institute for Clinical Excellence (Box 1), some psychiatrists remained reluctant to prescribe atypicals and, in some cases, had been restricted by pharmacy or health authority directives, owing to cost (Reference Taylor, Mir and MaceTaylor et al, 1999).
Box 1 Summary of NICE guidelines (2002) Footnote 1
-
• The choice of antipsychotic drug should be made jointly by the patient and responsible clinician, on the basis of an informed discussion of benefits and side-effects
-
• Oral atypical antipsychotics are recommended as first-line treatment for patients with newly diagnosed schizophrenia
-
• If a patient on oral typical antipsychotics has adequate symptom control but is experiencing unacceptable side-effects, an oral atypical should be considered
-
• If a patient on an oral typical has good symptom control and no unacceptable side-effects, a routine switch to an atypical preparation is not recommended
-
• Clozapine should be used at the earliest opportunity for patients with evidence of treatment-resistant schizophrenia
-
• A risk assessment should be performed regarding treatment adherence, and depot preparations should be prescribed when appropriate
-
• Where more than one atypical drug is considered appropriate, the drug with the lowest purchase cost (allowing for daily required dose) should be prescribed
-
• Where full discussion between the patient and responsible clinician is not possible, oral atypicals should be the treatment of choice because of the lower potential risk of extrapyramidal symptoms
-
• Antipsychotic therapy should be initiated as part of a comprehensive package of care that addresses the patient’s clinical, emotional and social needs
-
• Atypical and typical antipsychotics should not be prescribed concurrently, except for short periods to cover changeover of medication
However, many clinicians continue to promote the use of depot antipsychotics (Reference Glazer and KaneGlazer & Kane, 1992; Reference GerlachGerlach, 1995; Reference Kane, Aguglia and AltamuraKane et al, 1998). The relative efficacy and adverse effects of the various depot preparations have been examined and are summarised in a meta-review by Reference Adams, Fenton and QuraishiAdams et al(2001). Overall analysis of the many randomised controlled trials (RCTs) included in the review showed only a modest benefit for depot over oral antipsychotics. Such trials may not provide a complete picture; few were of a duration sufficient to obtain the maximum benefit in terms of relapse prevention. Reassuringly, there was little evidence that people receiving depot medication experienced greater side-effects than those taking oral preparations. However, acceptance of depot typical antipsychotics by patients and clinicians is variable and the mode of delivery seems to be a major stumbling block (Reference Walburn, Gray and GournayWalburn et al, 2001).
Underutilisation: clinicians’ fears and expectations
The main indication for depot antipsychotics is in maintenance treatment for people with schizophrenia for whom relapse prevention is indicated. For this target population it has been found that 50% are not treated prophylactically at all or only for an inadequate duration (Reference KisslingKissling, 1994, Reference Kissling1997). Prescribing practices for depot antipsychotics differ significantly between countries, with higher rates of depot prescribing in Denmark, Sweden and the UK, and lower rates in France and the USA (Reference Dencker and AxelssonDencker & Axelsson, 1996). Prescribing habits can also vary greatly within a region and between regions of one country (Reference Taylor, Mir and MaceTaylor et al, 1999). Further, the prescribing practice of an individual psychiatrist is subject to a multitude of influences, including the psychiatrist’s beliefs about adverse side-effects, the patient’s acceptance of depots, stigma, involvement of nursing staff, external forces in healthcare systems, prescribing knowledge and experience (Box 2).
Box 2 Tackling myths about depot drugs
-
• The risk of neuroleptic malignant syndrome is not higher for depot than oral drugs
-
• There is no evidence to suggest that neuroleptic malignant syndrome is a contraindication for subsequent depot use
-
• For the same drug, the risk of tardive dyskinesia is not higher for depot than oral formulations
-
• Patients already on depot like this formulation and many prefer depot to oral drugs
-
• Although depots are sometimes associated with coercion and reduced patient autonomy, coercion is not very common
-
• Clinicians perceive a stigma to be associated with depots but this may be based on the worst characteristics of typical drugs (e.g. unacceptable side-effects) rather than on intramuscular long-acting injections per se
-
• Most nursing staff are aware of the benefits of depots but their training experiences and pressure of time may adversely affect systematic monitoring of potential side-effects
-
• Depot clinics are relatively cheap to run and the financial benefits of avoiding rehospitalisation are clear-cut but acute services currently have preference in terms of service planning and budgeting
-
• Prescriber knowledge about depots may be suboptimal, resulting in use of inadequate dose and/or premature discontinuation of treatment, with subsequent poor clinical outcomes
Adverse side-effects
In comparison with conventional oral antipsychotics, some clinicians perceive depot preparations to be associated with an increased risk of certain adverse side-effects (Reference Kane, Aguglia and AltamuraKane et al, 1998) and neuroleptic malignant syndrome. The risk of the latter, however, has not been found to be higher for depot drugs, although the evidence base is weak (Reference Glazer and KaneGlazer & Kane, 1992). Further, Glazer & Kane stated that there are no data to suggest that a prior history of neuroleptic malignant syndrome is a contraindication to the use of depot antipsychotics.
Depot preparations are thought by some clinicians to cause tardive dyskinesia more frequently than oral preparations (Reference KisslingKissling, 1994; Reference Kane, Aguglia and AltamuraKane et al, 1998). Reference Adams, Fenton and QuraishiAdams et al(2001) did not confirm a greater risk of tardive dyskinesia in individuals receiving depot medication in RCTs, although this may be because adequate long-term studies have seldom been reported.
There is, perhaps, more of a consensus on extrapyramidal symptoms. It is generally agreed that typical antipsychotics (oral or depot) cause such symptoms more frequently and more severely than atypical antipsychotics. However, when comparing existing depot typical preparations with oral preparations of the same drug, there was no conclusive evidence of a difference (Reference Adams, Fenton and QuraishiAdams et al, 2001).
Patient acceptance
Some clinicians believe that the depot formulation is not a good treatment option because patients have negative attitudes towards parenteral administration. In a survey of out-patients by Reference Pereira and PintoPereira & Pinto (1997), 87% of those receiving depot antipsychotics (with or without oral augmentation) would, given a free choice, elect to continue with their present dose form. Similarly, Reference WistedtWistedt (1995) found that more than 60% of patients converted to depot medication preferred the injection to their previous tablet treatment and said that they felt better during the injection regimen. In a qualitative survey, patients on depot felt that ‘more normal lives’ are possible and that depots were a safety net protecting them from relapses and rehospitalisations (Reference Svedberg, Backenroth-Ohsako and LutzenSvedberg et al, 2003). In The Netherlands, out-patients receiving oral or depot antipsychotics were compared and were found to have similar attitudes towards their disease and medication use (Reference Hoencamp, Knegtering and KooyHoencamp et al, 1995). However, the patients were clearly biased towards the medication that they were receiving at the time.
Some patients prefer depot medication because the injections are easier to remember than daily tablets (Reference WistedtWistedt, 1995). Indeed, patients who have a tendency to deny their illness may be more comfortable with monthly or fortnightly injections than having to remember to take tablets daily. Others, however, fear the pain of intramuscular injections or consider injections to be intrusive or degrading (Reference Glazer and KaneGlazer & Kane, 1992). Reference Walburn, Gray and GournayWalburn et al(2001) conducted a systematic review of satisfaction with depot antipsychotic medication. In total, twelve main studies were considered: in ten of these, a positive opinion towards depot antipsychotics was expressed, in one a neutral opinion and in one a negative opinion. Five out of six studies that compared depot with oral antipsychotics showed patient preference for depots, although again, patients tended to state a preference for the formulation that they were taking at the time. Furthermore, in all of these studies it is only possible to present the opinions of patients who are willing to engage in the research process; as non-adherent patients rarely participate, their opinions are not necessarily represented. In this age of increasing consumer choice and decreasing medical paternalism, where concordance is preferred over compliance, the real issue is to ensure that a representative view of patients is obtained.
Stigma
A generally negative attitude towards ‘chemical’ treatments of mental diseases is sometimes found among patients’ families and friends (Reference GerlachGerlach, 1995; Reference KisslingKissling, 1997). When considering depot antipsychotics, Reference Pereira and PintoPereira & Pinto (1997) stated that
‘“Consumer advocates” concentrate on the undeniable adverse effects of antipsychotic drugs and upon the accusation that depot treatments involve an element of coercion’.
In society at large, both public opinion and the media often support these views and throw suspicion on professions who use somatic means, especially depot injections, in their treatment of mental diseases. Much more information is needed for patients, relatives and the public to engender more balanced attitudes (Reference GerlachGerlach, 1995).
Some psychiatrists associate depot antipsychotics with ‘non-compliant’ or ‘bad’ patients (Reference Glazer and KaneGlazer & Kane, 1992), but non-adherence to prophylactic or maintenance treatments is not unique to schizophrenia and is seen in many medical specialties (Reference Cramer and RosenheckCramer & Rosenheck, 1998). Unfortunately, there are few published data on psychiatrists’ attitudes to depot medication in the era of oral atypical antipsychotics. Further, it is not known how many clinicians feel that there is a stigma attached to depot medication and that they do indeed represent a more coercive form of treatment. As noted above, prescribing practices for depots vary, and in the USA it has been reported that White patients are less likely to receive depot medication than Black and Hispanic patients (Reference Valenstein, Copeland and OwenValenstein et al, 2001). This is either evidence of bias against people from minority ethnic groups or preferential treatment, depending on one’s point of view. There is also the possibility that, given the wide and successful publicity promoting the atypical agents – at the expense of the typicals (oral and depot) – depots have taken on the supposed worst characteristics of typical antipsychotics, namely unacceptable side-effects and even ineffectiveness. On the other hand, other clinicians will have experienced the disappointment of having a patient respond well to oral atypicals only to discontinue them and relapse.
Nursing staff involvement
In their systematic review, Reference Walburn, Gray and GournayWalburn et al(2001) identified only a handful of studies that specifically investigated the nursing staff’s opinion of depot antipsychotics. Reference Bennett, Done and HuntBennett et al(1995) suggested that community psychiatric nurses (CPNs) had a positive attitude towards their involvement with medication, but they were monitoring their patients for only three or four side-effects. The Royal College of Nursing’s guidelines now advocate the use of systematic assessment tools for side-effect monitoring (Royal College of Nursing, 1994). Further investigation of nursing staff involvement in the administration of depot drugs found that treatment and care planning involving both patients and nurses is essential to enhance patients’ autonomy (and empowerment), which is a precondition for satisfactory nurse–patient interactions (Reference Marland and SharkeyMarland & Sharkey, 1999). The authors further commented that the method of drug administration should not influence the patient’s right to information. Thus, the role of nursing staff in depot administration should also include advocacy and education.
For primary care practice nurses (who, in general, receive little training in depot administration), knowledge of schizophrenia, its treatment and medication side-effects are often poor. Practice nurses in one survey reported avoiding asking patients questions for fear of ‘opening a can of worms’. One nurse commented, ‘If they can’t be bothered to turn up, I can’t be bothered to chase them’ (Reference Kendrick, Millar and BurnsKendrick et al, 1998). It is hoped that specific training may lead to more positive attitudes.
External forces in healthcare systems: the plight of the depot clinic
Maintenance therapy is usually regarded as secondary to treatment of acute symptoms in terms of planning and financial budgeting, to the detriment of maintenance and prophylactic services. Indeed, Reference Anderson, Leadbetter and WilliamsAnderson et al(1989) reported that the depot clinic was perceived as being ‘out of date, not geared to the needs of the patient, inaccessible and unable to provide personalised care’. The financial incentives of preventing rehospitalisation, however, are self-evident and maintenance services such as depot clinics are comparatively cheap to run (Reference Remington and AdamsRemington & Adams, 1995). Reference O'Ceallaigh and FahyO’Ceallaigh & Fahy (2001) suggested that the currently less fashionable depot clinic be renamed ‘maintenance medication clinic’ and include patients receiving oral atypical maintenance medication. Systematic economic studies should be undertaken to evaluate maintenance treatments such as depot antipsychotics and associated services to guide future planning.
The impact of prescribing knowledge and experience
Owing to the large variety of antipsychotics now available, the choice of maintenance medication has become more complicated. In a naturalistic study conducted in Slovenia, the best predictor for prescription of depot antipsychotics was previous use of depot formulations by the individual patient (Reference Tavcar, Dernovsek and ZvanTavcar et al, 2000). So there is a positive cycle of familiarity and continued use. Depot medication is often regarded as useful only in those patients who are at high risk of non-adherence to treatment (Reference Barnes and CursonBarnes & Curson, 1994; Reference Davis, Matalon and WatanabeDavis et al, 1994). Some clinicians may be largely unfamiliar with current prescribing guidelines for depot antipsychotics (Reference KisslingKissling, 1994; Reference Dencker and AxelssonDencker & Axelsson, 1996) and therefore use inadequate doses or discontinue treatment too rapidly, or even prematurely, resulting in unfavourable outcomes in terms of relapse prevention.
Advantages of depot medication
Depot antipsychotics have several advantages over oral medication, including improved treatment adherence and hence reduced treatment failures, guaranteed and consistent delivery of the drug and reduced risk of side-effects and overdose (Box 3).
Box 3 Advantages of depot antipsychotics over oral preparations
-
• Improved treatment adherence, especially by overcoming covert non-adherence
-
• Easier early detection of relapse, improved relapse prevention and reduced rehospitalisation rates
-
• Enhanced consistency between the drug prescription and drug delivery
-
• More predictable and stable serum concentrations of the active drug
-
• Less variability between patients in steady-state blood levels for a given dose
-
• Lowest effective dose principle more safely achieved with depots (step-wise reduction)
-
• Reduced risk of accidental or deliberate self-poisoning (overdose)
Improved treatment adherence and relapse prevention
Depot antipsychotics are generally considered to improve overall rates of treatment adherence with regard to consistency between the drug prescription and drug delivery, and the necessary addition of recordable and regular staff–patient contact. Patients not managed under a compulsory treatment order, however, may accept or refuse depot medication in the same way as any other treatment. Depot formulations do not overcome the problem of a patient failing or refusing to attend the clinic for their medication (Reference Glazer and KaneGlazer & Kane, 1992; Reference Barnes and CursonBarnes & Curson, 1994; Reference GerlachGerlach, 1995). In such cases, the clinical team can respond appropriately to reduce the risk of relapse by actively trying to re-engage the patient (Reference Weiden and GlazerWeiden & Glazer, 1997). Hence, depot antipsychotic medication largely overcomes the problem of covert non-adherence and enhances clinical management for overt non-adherence. Further, it should be noted that poor treatment adherence can in itself be a sign of impending relapse rather than a consequence (Reference Curson, Barnes and BamberCurson et al, 1985). Depot antipsychotics are unable to prevent relapse completely; even in clinical trials there is an irreducible 20–25% of patients who relapse, despite receiving depots (Reference Adams, Fenton and QuraishiAdams et al, 2001). If a patient discontinues depot treatment their risk of relapse increases, but if a patient relapses despite regular depot injections then non-adherence can be safely excluded as the cause (Reference Barnes and CursonBarnes & Curson, 1994).
While it might be assumed that improved adherence to medication regimens will inevitably lead to reduced rates of relapse, demonstrating this in rigorous RCTs is not simple (Reference Adams, Fenton and QuraishiAdams et al, 2001). Yet evidence from other study designs, such as ‘mirror image’ studies – which may or may not involve random allocation to treatment – and in which outcomes are compared within individuals before and after introduction of depot medication, seems unequivocally in favour of depots (Reference Davis, Matalon and WatanabeDavis et al, 1994).
Consistent drug delivery
Depot formulations allow more predictable and stable serum concentrations of the active drug than do oral formulations and may increase the likelihood that the blood levels remain in the therapeutic range over long periods (Reference Glazer and KaneGlazer & Kane, 1992; Reference Barnes and CursonBarnes & Curson, 1994; Reference Remington and AdamsRemington & Adams 1995). Owing, it is presumed, to the avoidance of first-pass metabolism by the gastrointestinal system and liver, depot antipsychotics are also associated with less variability between patients in steady-state blood serum levels for a given dose than are oral formulations (Reference Glazer and KaneGlazer & Kane, 1992; Reference GerlachGerlach, 1995; Reference Darby, Pasta and DabiriDarby et al, 1995). Notably, slight peaks in serum levels of the active drug have been observed shortly after depot administration and patients report an increase in side-effects (e.g. sedation) during the first few days after receiving their injections. However, the overall long-term benefits in avoiding daily oscillations related to the repeated ingestion of oral medication have to be balanced against this.
Reduced risk of side-effects
Clinicians are familiar with the concept of risk–benefit analysis when considering which dose to prescribe. At low depot drug doses, the clinical benefits are reduced and the risk of relapse is higher, whereas at high doses the probability of disabling side-effects is increased with limited benefits in terms of symptom improvement. Moderate doses are effective for most patients. Indeed, depot formulations are considered to provide a better and safer way to use the lowest effective dose principle to reduce the frequency of side-effects, as a gradual step-wise reduction can be achieved without incurring a significant risk of severe relapse. Lengthening the injection interval is also a safe, alternative method for reducing the overall dose of depot atypical antipsychotics (Reference Baldessarini, Cohen and TeicherBaldessarini et al, 1988; Reference GerlachGerlach, 1995; Reference Carpenter, Buchanan and KirkpatrickCarpenter et al, 1999).
Reduced risk of overdose
It should also be remembered that deliberate or accidental self-poisoning with antipsychotic medication is avoided by depot prescription (Reference Dencker and AxelssonDencker & Axelsson, 1996). This important outcome measure is seldom investigated in clinical trials.
What will encourage physicians to prescribe depot antipsychotics?
In our surveys of attitudes to depot typical antipsychotic treatment held by psychiatrists and CPNs in the south-east of England, we found that a substantial minority believe that depots are old-fashioned (40% and 34% respectively), stigmatising (48% and 44%) and produce more side-effects than the oral typical antipsychotics (38% and 54%). The majority believe that depots are less acceptable to patients (69% and 61%) and their relatives (66% and 49%), but are better for monitoring treatment adherence (81% and 99%) and relapse prevention (94% and 89%) (Reference Patel, Nikolaou and DavidPatel et al, 2003a ,Reference Patel, Nikolaou and David b , Reference Patel, deZoysa and Baker2005). Compared with psychiatrists, significantly more CPNs felt that a patient’s autonomy was more compromised if they received a depot and also believed that prescribing depots was more coercive. Alternatively, psychiatrists were much more likely than CPNs to consider local inflammation at the injection site to be a rare event.
Psychiatrists also reported they could be persuaded to prescribe depot antipsychotics if they were associated with fewer side-effects, in patients where treatment adherence is an issue and if depot atypical antipsychotics were available. With the anticipated change in legislation in the UK to allow for community treatment orders, a significant majority of psychiatrists surveyed would use the legislation to continue depot administration with the aim of preventing further morbidity. Certainly the need for depot formulations continues and the first depot atypical (risperidone long-acting injection) shows promise, with efficacy equivalent to that of oral risperidone and a similar side-effect profile (Reference Altamura, Sassella and SantiniAltamura et al, 2003; Reference Fleischhacker, Eerdekens and KarcherFleischhacker et al, 2003; Reference Kane, Eerdekens and LindenmayerKane et al, 2003). Depot risperidone uses microsphere technology rather than the traditional esterification. Risperidone microspheres have different pharmacokinetics, requiring two or three fortnightly injections before therapeutic levels are reached.
A recent Australian survey highlighted that the lack of depot atypical preparations was the major reason for the common but generally frowned-upon practice of concurrent depot-plus-oral atypical prescribing in forensic patients (Reference Bains and NeilssenBains et al, 2003). Now that the first of the depot atypicals is available, these drugs may simplify the formulation dichotomy between typicals and atypicals (Reference Tavcar, Dernovsek and ZvanTavcar et al, 2000; Reference O'Ceallaigh and FahyO’Ceallaigh & Fahy, 2001) (Box 4).
Box 4 Antipsychotic drugs – class, formulation and characteristics
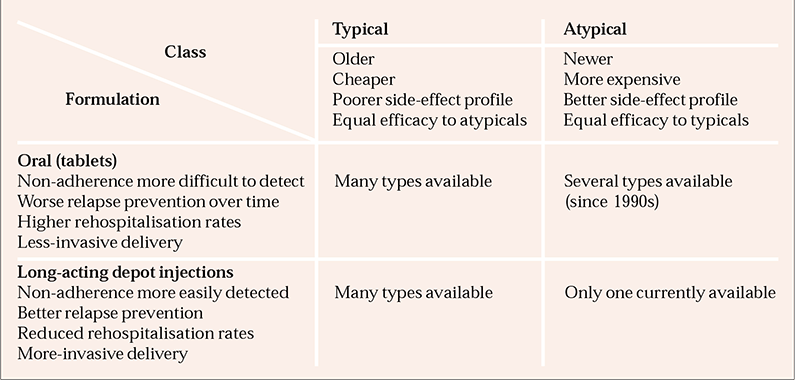
| Class | Typical | Atypical |
|---|---|---|
| Older | Newer | |
| Cheaper | More expensive | |
| Formulation | Poorer side-effect profile | Better side-effect profile |
| Equal efficacy to atypicals | Equal efficacy to typicals | |
| Oral (tablets) | ||
| Non-adherence more difficult to detect | Many types available | Several types available |
| Worse relapse prevention over time | (since 1990s) | |
| Higher rehospitalisation rates | ||
| Less-invasive delivery | ||
| Long-acting depot injections | ||
| Non-adherence more easily detected | Many types available | Only one currently available |
| Better relapse prevention | ||
| Reduced rehospitalisation rates | ||
| More-invasive delivery | ||
In our studies we found significant correlations between attitudes to depot medication and knowledge about depots. General knowledge was associated with more favourable attitudes (psychiatrists: r = 0.39, P<0.001; CPNs: r = 0.33, P = 0.006) (Reference Patel, Nikolaou and DavidPatel et al, 2003a , Reference Patel, deZoysa and Baker2005). These associations do not necessarily show causality. However, Reference Lambert, Brennan and CastleLambert et al(2003), in their survey of Australian mental health professionals, noted that potential barriers for switching to depot antipsychotics included fear of relapse as well as uncertainty of switching method, both of which could be addressed by augmenting clinicians’ knowledge about depots. To date, suggestions to improve this state of affairs, for example using publications, conferences and continued medical education, have had little impact.
Conclusions
At present, depot antipsychotics have an image problem, even though patients already receiving depots like them, as do many psychiatrists, and with good reason. The depot formulation has several advantages over oral medication, including improved treatment adherence and consistent drug delivery. Reference Glazer and KaneGlazer & Kane (1992) stated that if psychiatrists ‘want depot medication to work, they must weigh the negative aspects against the positive ones and believe in what they are doing’. We would add that this must be a collaborative process with patients and carers. Updating psychiatrists’ knowledge about depot formulations may lead to more positive attitudes. Similarly, educating patients’ relatives, friends and the general public may result in a more balanced attitude to antipsychotic medication in general, as well as to depot injections in particular. This may result in pressure on psychiatric healthcare systems to re-evaluate their maintenance therapy services in terms of both planning and budgeting. However, with the likely impact of the community treatment order on depot use, it is perhaps inevitable that the parenteral route of administration will always have associations with coercion. Future research should therefore address the issues of coercion, stigma and patient autonomy.
MCQs
-
1 Depot injections:
-
a overcome covert non-adherence
-
b are administered every 1–6 weeks
-
c are administered subcutaneously
-
d enhance relapse prevention
-
e are available only for typical antipsychotics.
-
-
2 Treatment adherence for antipsychotics:
-
a is considerably worse than that for other drugs and other illnesses
-
b is dependent on the patient’s health beliefs
-
c is unrelated to the patient’s personal opinion regarding their susceptibility to relapse
-
d is always accurately predicted by a patient’s verbal report of their adherence behaviour
-
e is mainly dependent on the drug’s side-effect profile.
-
-
3 Reasons for depot underutilisation include:
-
a depot clinics are expensive to run
-
b patients naturally prefer oral to depot formulations
-
c depots have an image problem
-
d depots are associated with coercion
-
e suboptimal prescriber knowledge regarding these drugs.
-
-
4 Advantages of depot antipsychotics (compared with oral) include:
-
a easier early detection of relapse
-
b reduced consistency between the drug prescription and drug delivery
-
c more variability between patients in steady-state blood levels for a given dose
-
d reduced rehospitalisation rates
-
e reduced risk of deliberate self-poisoning.
-
-
5 Regarding the future use of depot antipsychotics:
-
a depot utilisation rates will be affected if new legislation includes a community treatment order
-
b availability of atypical depot antipsychotics will have no impact on depot prescribing
-
c prescribers require more information regarding switching to depots
-
d NICE does not advocate the use of typical depot antipsychotics
-
e the decision to switch to a depot should be openly discussed with the patient and carer beforehand.
-
MCQ answers
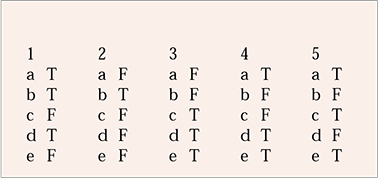
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | T | a | F | a | F | a | T | a | T |
| b | T | b | T | b | F | b | F | b | F |
| c | F | c | F | c | T | c | F | c | T |
| d | T | d | F | d | T | d | T | d | F |
| e | F | e | F | e | T | e | T | e | T |





eLetters
No eLetters have been published for this article.