Medically unexplained physical symptoms are common among healthy people and those attending clinics. About 20–30% of people seen in general practice lack a demonstrable cause for their symptoms (Reference Rosendal, Olesen and FinkRosendal et al, 2005) and the figures are higher still in general hospitals (Reference TalleyTalley, 1998; Reference Stone, Zeman, Halligan, Bass and MarshallStone & Zeman, 2001). Although liaison psychiatrists encounter some of these patients, other clinicians manage most of them, confronting the attendant risks of over-investigation, misdiagnosis and inappropriate treatment. Many psychiatric syndromes can be associated with unexplained physical phenomena (Box 1), and these vary greatly in their phenomenology, proposed aetiology and appropriate management. What these syndromes share is an irreducible, subjective core: only the patient can fully describe their symptom, there is no external reference; that is, with symptoms such as pain or somatic hallucination, there is nothing for the physician ‘to see’ (Reference BerriosBerrios, 1982).
Box 1 Some psychiatric syndromes that have an impact on bodily experience (ICD–10 diagnostic codes are in parentheses)
-
• Paranoid schizophrenia (F20.0) – passivity phenomena, somatic hallucinations
-
• Schizoptypal personality (F21) – bodily illusions, dysmorphophobic ruminations
-
• Persistent delusional disorder (F22) – hypochondriacal (somatic) delusions, dysmorphophobia, delusional parasitosis
-
• Severe depression with psychotic symptoms (F32.3) – hypochondriacal delusions, Cotard's delusion
-
• Dissociative (conversion) disorder (F44) – includes motor signs (44.4), convulsions (44.5) and sensory loss (44.6)
-
• Somatoform disorder (F45) – includes somatisation disorder (45.0) and hypochondriacal disorder (45.2) – would also subsume Briquet's syndrome
-
• Persistent somatoform pain disorder (F45.4)
-
• Neuraesthenia (F48) – fatigue syndromes
-
• Anorexia nervosa (F50.0) – disturbed body image
It may seem paradoxical to set about investigating the physical basis of symptoms that are regarded as ‘medically unexplained’ since their classification hinges upon their having no explanation and, hence, by implication, no biological basis. However, such a view fails to acknowledge the constant interaction between ‘states of mind’ and ‘states of the body’ in a cognitive neurobiological system – the brain. It also risks minimising the patient's reality, a lived experience that is ‘embodied’ (Reference Butler, Evans and GreavesButler et al, 2004). Whether the symptom is numbness or fatigue, awareness of that symptom and its emotional sequelae are proposed to be represented within the central nervous system, and even a deliberately feigned disorder depends on neurological processes for its ‘performance’ (Reference Spence, Crimlisk and CopeSpence et al, 2000). Hence, although it might be reasonable to state that some disorders lack a demonstrable lesion (e.g. a tumour), it seems unlikely that they will lack any relationship to the state of the patient's brain.
Cognitive neurobiological architecture
Before embarking on a review of the sites of dysfunction implicated in specific disorders it is appropriate to hypothesise where such symptoms might arise. Which brain systems represent the ongoing state of the body? This is a complex question and is the subject of much contemporary research.
In simple terms, there is a distinction between the frontal lobes of the brain, anterior to the central sulcus, and the posterior cortices: the parietal, temporal and occipital lobes. The latter receive incoming sensory information from the major sense organs, via the thalamus, whereas the frontal systems are responsible for predicting, planning, programming and executing actions in the world (and also in ‘the mind’; Reference Spence and FrithSpence & Frith, 1999). All these systems are interconnected, so that even a simple act is the product of motor initiation, sensory expectation and feedback. Subcortical structures also play an important role: the frontal lobes contain at least five semi-closed loops, which funnel information from specific cortical fields to targeted regions of the striatum (caudate and putamen), on into the thalami, whence they are closed by further projections, returning to the cortex (Reference Alexander and CrutcherAlexander & Crutcher, 1990). The ‘motor’ loop focuses primarily on the supplementary motor area, an area of premotor (programming) cortex high up on the medial surface of the frontal lobes; the ‘executive’ loop projects to the dorsolateral prefrontal cortex, an area implicated in working memory, planning and novelty generation. The latter circuit also incorporates the caudate nucleus, a component of the striatum that is involved in planning, reward and emotional processing (Fig. 1).
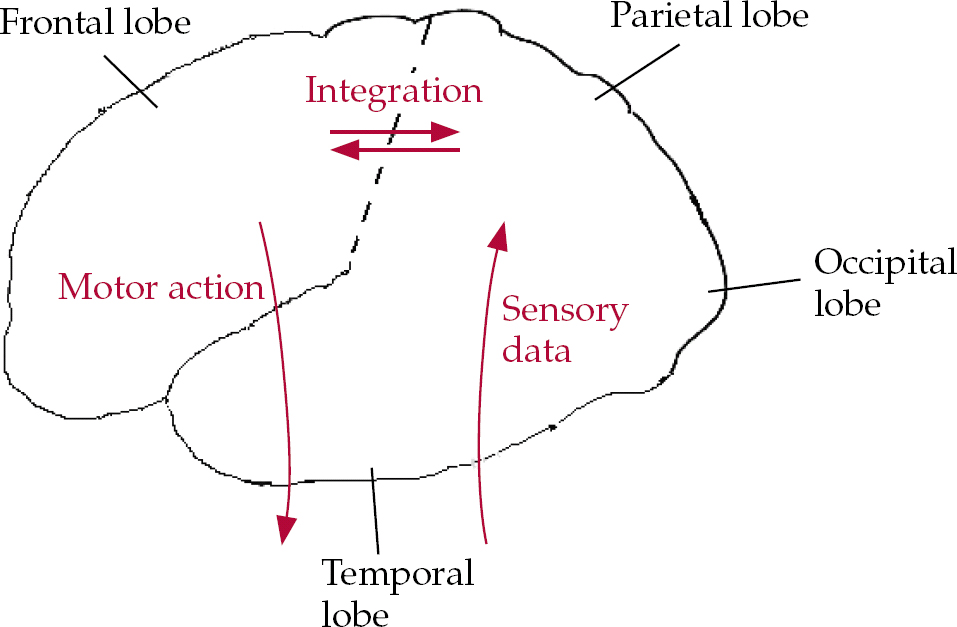
Fig. 1 Cartoon illustrating a much-simplified cognitive architecture of the frontal and posterior brain systems’ utilisation of incoming sensory data. The initiation of voluntary actions implicates frontal systems (see text for details).
Although the sense organs send data to the primary sensory areas in the parietal (somatosensory), temporal (auditory) and occipital (visual) cortices for preliminary analyses, more complex computations occur elsewhere. Hence, there are streams of data leaving the occipital cortices along the ventral (‘what is it?’) and dorsal (‘where is it?’) pathways, to the temporal and parietal cortices respectively. Within the temporal cortices there are regions concerned with semantic knowledge – the identity and meaning of stimuli. There are heteromodal regions of the parietal cortex that are engaged in the analysis and synthesis of somatic, auditory and visuospatial information with reference to egocentric, bodily oriented coordinates.
We might predict that certain disorders of bodily experience would involve such systems, particularly those within the parietal and temporal cortices (i.e. those that may be grossly simplified as dealing with bodily ‘space’ and ‘meaning’ respectively). We might also be more specific and differentiate somatic (pertaining to bodily surface and voluntary musculature) from visceral (bodily interior and involuntary musculature) sensations. It has been suggested that one reason visceral (as opposed to somatic) sensations are so difficult to localise within the body is that their projections are not focused on the primary somatosensory strip (with its familiar homunculus, representing localised somatic sensation) but instead more diffusely distributed, primarily to the secondary somatosensory cortices and limbic regions (Reference Schnitzler, Volkmann and EnckSchnitzler et al, 1999; Reference Aziz, Schnitzler and EnckAziz et al, 2000). This is illustrated by the pain associated with appendicitis: vaguely central abdominal (visceral) pain precedes specific localisation of pain to the right iliac fossa, following peritoneal (somatic) involvement.
Furthermore, the simple association of frontal regions with motor systems and posterior regions with sensory systems breaks down when we consider emotional states of the body. Medial frontal regions (including the anterior cingulate cortex) are particularly concerned with subjective awareness of pain and emotional experience (Reference Peyron, Laurent and Garcia-LarreaPeyron et al, 2000; Reference Phan, Wager and TaylorPhan et al, 2002), whereas the basic emotions, such as fear and disgust, involve other limbic foci (in particular amygdala and caudate respectively) (Reference Calder, Lawrence and YoungCalder et al, 2001; Reference Phan, Wager and TaylorPhan et al, 2002). Such systems modulate autonomic arousal and responsivity and are implicated in conditions such as panic disorder and obsessive–compulsive disorder (OCD). Emotional processing also has an impact on relationships with others because it is linked to the ability to recognise their distress. This suggests that amygdala and medial prefrontal dysfunction might underlie certain aspects of psychopathy (Reference DamasioDamasio, 2000; Reference BlairBlair, 2003).
Imaging techniques
Magnetic resonance imaging (MRI) provides a safe means of assessing the structure of the living brain. Other techniques can detect local or diffuse changes in brain activity. They include those that utilise radiotracers (positron emission tomography – PET – and single-photon emission computed tomography – SPECT) and those that use MRI (such as functional magnetic resonance imaging – fMRI). These techniques are often used to detect proxy markers of synaptic activity; for example, changes in regional cerebral blood flow in certain forms of PET and SPECT, and changes in blood oxygen level-dependent response in fMRI. Techniques that use radiotracers (PET and SPECT) may also be used to measure regional brain metabolism (e.g. with radiolabelled glucose molecules) and neurotransmitter receptor binding (with radiolabelled ligands). Hence, there is a wide range of methods that may be applied in the investigation of the biology of neuropsychiatric disease, although these vary in their availability and the ease with which they can be used in non-specialised centres.
Syndromes affecting bodily experience
Passivity phenomena
Central to the concept of schizophrenia is a breach of physical borders, which we often interpret in mental terms: the dissolution of ego boundaries exemplified by the Schneiderian first-rank symptoms (Reference Schneider, Hirsch and ShepherdSchneider, 1974). The patient with passivity phenomena, ‘made’ thoughts or movements, believes that their autonomy is being usurped by outside forces (Reference Mullins and SpenceMullins & Spence, 2003). A spirit or persecutor controls their movements. Their thoughts are directly accessed by others.
From which brain systems might this sensation arise? Study of patients with neurological damage has suggested that damage to the parietal lobes might produce similar phenomena (Reference SpenceSpence, 2001; Reference Spence and HalliganSpence & Halligan, 2002). One important theoretical account of first-rank symptoms attributes them to a failure in the monitoring of ongoing action; more specifically, to a failure by the brain's motor programming systems to predict the future sensory consequences of action (Reference Frith, Blakemore and WolpertFrith et al, 2000). By ‘forward modelling’, the brain predicts its own outcome states following future acts. If I lift my arm, my brain has already computed the sensory stimuli arising from such an act. A deviation from such predictions alerts the brain to potential errors and the need for correction. However, if there is a fault within the system then my action might feel as if it had come from ‘nowhere’, as if an outside force were responsible.
Although sophisticated cognitive psychological experiments have investigated whether people experiencing first-rank symptoms are aware of their actions (Reference Frith and DoneFrith & Done, 1989), one of the advantages of functional neuroimaging techniques is that they allow investigators to examine the motor system directly and to locate functional abnormality. In one such study, Reference Spence, Brooks and HirschSpence and colleagues (1997) used PET to examine the brain activity associated with moving a joystick in freely chosen directions by people with schizophrenia experiencing passivity (‘made’ thoughts and actions), other people experiencing psychosis but not passivity phenomena, and healthy controls. Patients with passivity exhibited relative hyperactivation of the right inferior parietal cortex, an area implicated in spatial awareness and programming, and the left supplementary motor area, a region of premotor cortex that is also involved in the programming of action. Hence, the findings are consistent with the view that systems engaged in programming action in space are disturbed in people experiencing passivity (their disturbance remitting with symptomatic recovery) (Reference Spence, Brooks and HirschSpence et al, 1997).
Subsequent studies have also found parietal abnormalities in patients with such symptoms (Reference Franck, O'Leary and FlaumFranck et al, 2002). In one recent study hyperactivation of the right parietal cortex was found while such patients performed self-paced finger movements in an MRI scanner (Reference Ganesan, Hunter and SpenceGanesan et al, 2005; Fig. 2). Another study has shown that people with schizophrenia reporting motor passivity phenomena (‘made’ movements) exhibit reductions in right parietal and left prefrontal grey matter volume relative to other people with schizophrenia (Reference Maruff, Wood and VelakoulisMaruff et al, 2005). Moreover, a study of students with high schizotypy scores revealed that those who feared losing control of their thoughts had reduced grey matter volume of the supplementary motor area (Reference Matsui, Yoneyama and SumiyoshiMatsui et al, 2002).

Fig. 2 Aberrant hyperactivation of right parietal (sensory) regions (Brodmann area 40) during action initiation by people experiencing first-rank symptoms of schizophrenia (Talairach coordinates = 57, −29, 36; T = 4.7; P < 0.0001 (uncorrected); family-wise error corrected P = 0.015). Data derived from Reference Ganesan, Hunter and SpenceGanesan et al 2005.
Hence, current studies on passivity phenomena suggest that the parietal cortices (which are concerned with spatial and bodily awareness) and key planning and programming areas within the frontal lobes are implicated in the disturbed sense of agency and bodily awareness.
Somatic hallucinations and delusions
An fMRI study of a 36-year-old man with schizophrenia who was experiencing somatic hallucinations (that he was ‘touched by spirits’) revealed an association with activation of corresponding primary somatosensory and posterior parietal cortices (Reference Shergill, Cameron and BrammerShergill et al, 2001). These findings were consistent with those of an earlier electroencephalographic study of a similar patient (Reference Baldeweg, Spence and HirschBaldeweg et al, 1998). Again, the suggestion is that sensory and association cortices are implicated in the generation of complex abnormalities of bodily experience encountered in schizophrenia. Note that the patient's phenomenology implicated the surface of the body (‘touched by spirits’), which is in line with the primary somatosensory cortex being activated.
There are several interesting case reports from Japan on neuroimaging in patients with delusional parasitosis. Reference Maeda, Yamamoto and YasudaMaeda and colleagues (1998) described an elderly man who felt worms moving around in his mouth. His MRI scan revealed an old infarction of the right putamen (an area receiving parietal afferents); his symptoms resolved with pimozide. Reference Wada, Kawakatsu and KomataniWada et al(1999) performed a SPECT investigation before and after response to clomipramine of an elderly woman who believed ‘something was moving in her abdomen’. At the height of her symptoms she exhibited hypoperfusion of left temporo-parietal cortices.
Similarly, Reference Ota, Mizukami and KatanoOta and colleagues (2003) used SPECT to investigate the brain function of an elderly man before and after modified electroconvulsive therapy for somatic (nihilistic) delusions: ‘Food disappears inside of my oesophagus’, ‘my jaw comes off’. This man exhibited hypoperfusion of left parietal and temporal regions, which improved following treatment. The same regions were implicated in an elderly woman who complained of oral infestation, which responded to paroxetine (Reference Hayashi, Oshino and IshikawaHayashi et al, 2004).
Patients with Cotard's delusion of negation are usually depressed and come to believe that they are dead or decaying. There have been rather disparate accounts of functional anatomical correlates of Cotard's delusion using SPECT. Reference Hashioka, Monji and SasakiHashioka et al(2002) described a patient with bilateral hypofrontality, which resolved on recovery; Reference De Risio, De Rossi and SarchiaponeDe Risio and colleagues (2004) found no focal perfusion deficit in a patient with more classical symptomatology (‘he had no internal organs and was already dead’) but did find a reduction in striatal D2 receptor binding, which is consistent with excessive endogenous dopamine at the time of psychosis. Reference Gardner-Thorpe and PearnGardner-Thorpe & Pearn (2004) described two further patients: one with associated arteriovenous malformations in the parietal lobes and the other with multiple sclerosis. Despite multiple sclerosis of 20 years’ duration the latter patient described recent onset of transient psychotic episodes. During such episodes she felt as if ‘her flesh were falling off’, ‘as if there were liquid inside her that was draining out’. ‘Whilst experiencing the symptom she believed it was really happening but next morning had gained a somewhat perplexed insight into its hallucinatory nature’ (Reference Gardner-Thorpe and PearnGardner-Thorpe & Pearn, 2004: p. 564). This case report exemplifies the blurring of phenomenological boundaries seen in certain accounts of somatic hallucinations, Cotard's delusion and, indeed, hypochondriacal delusions.
Dysmorphophobia
There is little recent work devoted to the onset of disturbed bodily awareness (dysmorphophobia), where the central feature is preoccupation with slight or non-existent defects in appearance, causing significant distress or impairment in functioning. About half of patients with body dysmorphic disorder experience psychosis, although there is also a phenomenological similarity to OCD, exemplified by repeated checking and ritualised behaviours. Reference Gabbay, Asnis and BelloGabbay and colleagues (2003) described a 24-year-old man who developed a preoccupation with the width of his nose following encephalitis; structural MRI revealed marked left-sided fronto-temporal atrophy.
The emergence of compulsive or ritualistic behaviours has been linked to both temporal lobe and basal ganglia pathology. There is a theory that specific basal ganglia circuits might be involved in OCD-spectrum disorders, with those disorders with cognitive phenomenology (OCD and body dysmorphic disorder) involving the caudate and those characterised by motor abnormalities (Tourette syndrome and trichotillomania) the putamen (Reference Rauch, Phillips and SegalRauch et al, 2003). In this regard it is of interest that an MRI study of eight middle-aged women with body dysmorphic disorder revealed leftward shift of caudate asymmetry and increased white matter volume. However, apart from being a marker of striatal abnormality it is unclear how such an asymmetry might disturb bodily awareness. A SPECT study of brain function in six people with body dysmorphic disorder found a broad range of abnormalities that implicate the parietal lobes (Reference Carey, Seedat and WarwickCarey et al, 2004). This is consistent with the role of the latter in supporting bodily awareness.
Eating disorders
Concern with the body's size and shape is a hallmark of the eating disorders, aspects of which seem to imply a misperception of bodily form. Is there evidence in favour of abnormal cerebral processing of the embodied self? Neuroimaging studies in people with eating disorders are surprisingly few, but already there are some interesting leads.
A study of three people with anorexia nervosa who were confronted with images of their own bodies revealed right amygdala activation, which is consistent with the latter's role in processing negative affect (Reference Seeger, Braus and RufSeeger et al, 2002). Although this finding awaits replication, a later study of a larger sample revealed that people with anorexia and/or bulimia exhibited reduced activation of right temporo-parietal regions (those concerned with bodily awareness and recognition), in comparison with healthy controls, when confronted with images of standard female forms (Reference Uher, Murphy and FriederichUher et al, 2005). The authors concluded that functional abnormalities in specific regions might underlie abnormal self-perception in these disorders. The importance of right-hemisphere abnormalities in eating disorders is also supported by a recent review. Reference Uher and TreasureUher & Treasure (2005) report that although simple changes in appetite and eating behaviour occur with hypothalamic and brain-stem lesions, more complex disturbances, resembling the psychopathology of idiopathic eating disorders, arise more commonly following right frontal and temporal damage.
Recent PET studies of people recovering from anorexia have revealed abnormalities of central serotonergic and dopaminergic function (Reference Frank, Kaye and MeltzerFrank et al, 2002, Reference Frank, Bailer and Henry2005).
Conversion syndromesFootnote †
Conversion syndromes are characterised by abnormalities of voluntary movement and subjective report (e.g. hemi-anaesthesia) that cannot be explained by demonstrable cerebral pathology. The onset of the disorder is said to coincide with emotional distress and the symptoms themselves are inconsistent (e.g. paralyses may wax and wane, normal function may be elicited by distraction or sedation; Reference SpenceSpence, 1999). To date, there have been a few neuroimaging studies of single patients or small series of patients with conversion syndromes using SPECT, PET or fMRI. Results may have been influenced by confounding variables such as the presence of comorbid diagnoses (often depression and/or personality disorder) and symptom chronicity (not least since many such studies are from tertiary centres, which attract patients with atypical, chronic disorders). I have reviewed the pros and cons of these approaches elsewhere (Reference SpenceSpence, 1999, Reference Spence, Hallett, Fahn and Jankovic2006).
Nevertheless, a common theme has emerged (Box 2): sensory deficits (e.g. anaesthesia or blindness) are associated with hypoactivation of the relevant sensory cortices and evidence of disturbed function in ‘higher’ frontal regions (Reference Tiihonen, Kuikka and ViinamakiTiihonen et al, 1995; Reference Yazici and KostakogluYazici & Kostakoglu, 1998; Reference Vuilleumier, Chicherio and AssalVuilleumier et al, 2001; Reference Mailis-Gagnon, Giannoylis and DownarMailis-Gagnon et al, 2003; Reference Werring, Weston and BullmoreWerring et al, 2004); whereas motor disturbance is associated with either excessive activation of (inhibitory) orbitofrontal cortices (Reference Marshall, Halligan and FinkMarshall et al, 1997) or suppression of activation in the dorsolateral prefrontal cortex (an area involved in action generation) (Reference Spence, Crimlisk and CopeSpence et al, 2000). A common view is that lower centres are somehow inhibited or suppressed by higher centres. There has been debate as to whether these findings may adequately distinguish conversion syndromes from feigning (Reference Marshall, Halligan and FinkMarshall et al, 1997; Reference SpenceSpence, 1999). Nevertheless, there is the hope that functional neuroimaging techniques may (in the future) help to establish an empirical framework for conversion syndromes.
Box 2 Common themes from neuroimaging studies of conversion syndromes
-
• Sensory deficits (anaesthesia or blindness) are associated with hypoactivation of the sensory cortices and disturbed function of ‘higher’ frontal regions (Reference Tiihonen, Kuikka and ViinamakiTiihonen et al, 1995; Reference Yazici and KostakogluYazici & Kostakoglu, 1998; Reference Vuilleumier, Chicherio and AssalVuilleumier et al, 2001; Reference Mailis-Gagnon, Giannoylis and DownarMailis-Gagnon et al, 2003; Reference Werring, Weston and BullmoreWerring et al, 2004)
-
• Motor disturbance is associated with either excessive activation of (inhibitory) orbitofrontal cortices (Reference Marshall, Halligan and FinkMarshall et al, 1997) or suppression of activation in the dorsolateral prefrontal cortex (Reference Spence, Crimlisk and CopeSpence et al, 2000)
SomatisationFootnote ‡
Somatisation refers to recurrent, multiple somatic complaints which cannot be attributed to demonstrable organic pathology. It has been postulated to be a consequence of disturbed attention, so that people with somatisation are unable to filter out irrelevant somatic sensations. There are few neuro-imaging studies of people with somatisation.
Reference Garcia-Campayo, Sanz-Carrillo and BaringoGarcia-Campayo et al(2001) used SPECT to examine a series of people with somatisation who were without comorbid Axis I diagnoses. Visual inspection of the scans (there was no control group) revealed that most of their 11 participants had evidence of focal hypoperfusion, predominantly of the frontal, cerebellar and temporo-parietal regions. Subsequently, Reference Hakala, Karlsson and RuotsalainenHakala and colleagues (2002) used PET to compare brain function of women with chronic somatisation with that of a control group. The women with somatisation exhibited reduced metabolism of specific subcortical regions, including bilateral caudate nuclei. The volume of these caudate nuclei (in the same women) was found to be enlarged by MRI (Reference Hakala, Karlsson and RuotsalainenHakala et al, 2002, Reference Hakala, Karlsson and Kurki2004).
Chronic fatigue syndrome
Fatigue can be defined as difficulty in initiating or sustaining voluntary activities, and may be caused by central or peripheral neurological or systemic factors (Reference Chaudhuri and BehanChaudhuri & Behan, 2004). Chronic fatigue syndrome is characterised by a sense of fatigue that persists for at least 6 months and is unattributable to other physical or psychiatric disorder. It provides another example of a diagnosis of exclusion (similar to conversion and somatisation), resting very much on the patient's subjective account. Nevertheless, it is clinically desirable to perform structural MRI of the brain in patients with chronic fatigue, not least because persistent fatigue may be a presenting symptom of multiple sclerosis (Reference Lange, Wang and DelucaLange et al, 1998).
There have been many neuroimaging studies of people with chronic fatigue over the past decade and in recent years a range of scanning methods and techniques for data analysis have been used. Early studies suggested that structural, white matter defects might be more common in patients with chronic fatigue syndrome, particularly within the frontal lobes (Reference Natelson, Cohen and BrassloffNatelson et al, 1993; Reference Lange, Deluca and MaldjianLange et al, 1999; Reference Cook, Lange and DelucaCook et al, 2001), but this was not demonstrated by others (Reference Cope, Pernet and KendallCope et al, 1995; Reference Greco, Tannock and BrostoffGreco et al, 1997). The defects most often cited were so-called hyperintensities, possibly reflecting vascular pathology; these are often reported to increase with age in healthy people (and also to be over-represented in a number of psychiatric disorders).
Although focal functional deficits were also reported in early studies, a very well-controlled twin study of patients with chronic fatigue syndrome and their discordant (i.e. healthy) co-twins revealed no abnormalities of resting-state regional cerebral blood flow which were specific to the disorder (Reference Lewis, Mayberg and FischerLewis et al, 2001). Hence, there may be no trait resting-state functional abnormalities that adequately characterise all patients with chronic fatigue syndrome. Some investigators have refined their samples to focus on specific features of the disorder, e.g. late-onset syndrome or cognitive impairment. Others have focused on ‘cognitive challenges’: studying functional response while patients perform a cognitive task.
Reference Siessmeier, Nix and HardtSiessmeier and colleagues (2003) conducted the first PET study of cerebral metabolism in chronic fatigue in which the data were analysed using an observer-independent technique (i.e., not a manually determined region-of-interest analysis, which may be subject to observer bias). They found that, despite sample heterogeneity, participants demonstrated reduced activity in the orbitofrontal and anterior cingulate cortex, regions implicated in the control (inhibition and monitoring) of behaviour. However, there was no correlation with fatigue per se.
Reference De Lange, Kalkman and BleijenbergDe Lange et al(2004) used fMRI to study people with chronic fatigue who were attempting motor and visual imagery tasks. Under both conditions participants exhibited hypofunction of the caudate nuclei, whereas on error trials they failed to activate ventral regions of the anterior cingulate cortex (implicated in affect regulation).
In a structural MRI study, Reference Okada, Tanaka and KuratsuneOkada and colleagues (2004) used an automated, data-led approach to reveal bilateral reductions of cortical grey matter in dorsolateral prefrontal regions. The deficit in the right dorsolateral prefrontal cortex correlated with the severity of symptomatic fatigue. This area had also been implicated in an earlier SPECT study of late-onset chronic fatigue (Reference Goldstein, Mena and JouanneGoldstein et al, 1995).
Turning to neurochemistry, Reference Cleare, Messa and RabinerCleare et al(2005) summarised the evidence that fatigue may be associated with excessive serotonergic tone and demonstrated, using a ligand for the 5HT-1A receptor, that untreated patients without comorbid diagnoses had reduced 5HT-1A binding occupancy on PET scans. This finding would be compatible with reduced numbers of receptors or their decreased affinity for the ligand (which would be in accordance with a central disturbance of serotonergic function in chronic fatigue syndrome). There were no correlations with fatigue per se, although the patients studied were at, or close to, ceiling on their fatigue severity ratings. Hence, there may have been insufficient within-group variance to detect such a correlation.
Pain syndromes
Pain is processed within a distributed ‘pain matrix’ involving lateral systems, incorporating the lateral thalamic nuclei, insulae and secondary somatosensory cortices (concerned with pain localisation), and medial systems, incorporating the midline and intralaminar thalamic nuclei, amygdalae and anterior cingulate cortex (which are more concerned with its affective significance) (Reference Ladabaum, Minoshima and OwyangLadabaum et al, 2000; Reference Peyron, Laurent and Garcia-LarreaPeyron et al, 2000; Reference Jones, Kulkarni and DerbyshireJones et al, 2003; Reference VogtVogt, 2005). There is also a distinction between visceral and somatic pain: the former eliciting most response from within the medial system and the latter from the lateral system (Reference Dunckley, Wise and AzizDunckley et al, 2005). In general, visceral pain evokes greater ‘unpleasantness’ than somatic pain, even when ‘pain’ levels are controlled for. These phenomena differ in other respects: visceral pain elicits immobilisation whereas somatic pain is more likely to precipitate a fight/flight response (hence the greater involvement of motor regions) (Reference Ladabaum, Minoshima and OwyangLadabaum et al, 2000; Reference Dunckley, Wise and AzizDunckley et al, 2005, Reference VogtVogt 2005). Visceral pain is likely to be harder to localise (Reference Schnitzler, Volkmann and EnckSchnitzler et al, 1999).
Functional pain
The literature on functional pain syndromes such as irritable bowel syndrome, fibromyalgia and atypical facial pain has been reviewed by Reference DerbyshireDerbyshire (2003). A coherent message has yet to emerge, although there are interesting patterns. For instance, chronic functional pain might be associated with increased responsiveness of the anterior cingulate cortex, whereas chronic organic (specifically, inflammatory) pain might be associated with downregulation of the anterior cingulate cortex and thalamus (Reference Peyron, Laurent and Garcia-LarreaPeyron et al, 2000). In organic disease, adequate pain relief elicits increased activation of the latter foci (Reference Peyron, Laurent and Garcia-LarreaPeyron et al, 2000; Reference VogtVogt, 2005). The highest concentration of endogenous opioid receptors is found in the mid-anterior cingulate cortex, which is in accordance with this region's functional response to opiate analgesia and placebos (Reference Peyron, Laurent and Garcia-LarreaPeyron et al, 2000; Reference VogtVogt, 2005).
Angina pain
Angina pain is associated with activation of the anterior cingulate cortex and thalamus (and pre-frontal foci). However, ‘silent’ ischaemia activates the thalamus but does not activate the anterior cingulate cortex (Reference Ladabaum, Minoshima and OwyangLadabaum et al, 2000). This suggests that subjective awareness of pain is crucially linked to activity within the anterior cingulate cortex (consistent with its specific response to successful analgesia) (Reference Jones, Kulkarni and DerbyshireJones et al, 2003; Reference VogtVogt, 2005). It also suggests that the thalamus may play a role in gating the conscious perception of pain (Reference Ladabaum, Minoshima and OwyangLadabaum et al, 2000). Overactivity of the anterior cingulate cortex in functional pain syndromes may therefore reflect the role of higher centres, perhaps manifesting increased vigilance towards visceral sensation (Reference Aziz, Schnitzler and EnckAziz et al, 2000; Reference Ladabaum, Minoshima and OwyangLadabaum et al, 2000; Reference Derbyshire, Whalley and StengerDerbyshire et al, 2004). A detailed account of the anatomy of the anterior cingulate cortex and regional specialisation is provided by Reference VogtVogt (2005).
Conclusions
The advent of neuroimaging techniques has led to a great many studies aimed at discovering the biological correlates of a variety of somatic phenomena, from the frankly psychotic (e.g. delusional parasitosis) to the traditionally ‘neurotic’ (e.g. somatisation). At present, such studies are largely correlational and, although they implicate certain brain foci, they have yet to provide detailed accounts of pathophysiology.
Perhaps the most chastening lesson from this review is the striking lack of specificity of any of the findings so far described. Although we might predict that a patient describing bodily disturbance of ‘some kind’ will exhibit abnormalities of certain candidate brain regions, we would have great difficulty modifying their diagnosis or treatment on the basis of the results of brain scanning.
Also, the phenomenological boundaries between conditions are conspicuously porous (Reference Cahill, Frith, Halligan and MarshallCahill & Frith, 1996): for example, where does thought insertion end and somatic hallucination begin if a person can point to the site of entry of an alien thought? The comorbidity inherent in many of the physical syndromes makes it correspondingly difficult to obtain ‘pure’ samples (e.g. of people with conversion syndrome or somatisation); and, were they to be obtained, one might ask how representative they were of the population experiencing the syndrome. One might conclude that the diagnostic categories available to us do not constitute ‘natural kinds’. There is too much phenomenological overlap.
Hence, it is perhaps unsurprising that many of the same brain regions are implicated across different psychiatric syndromes. This survey reveals that the frontal lobes are often implicated where there is a failure of action, as in conversion disorder or chronic fatigue syndrome, whereas temporal and parietal lobes are often implicated in those disorders affecting the individual's awareness of their own subjective space (e.g. passivity phenomena or anorexia nervosa). Several disorders (body dysmorphic disorder, somatisation and chronic fatigue syndrome) implicate dysfunction of the caudate nuclei, areas of the striatum contributing to cognitive, motor and emotional control. A full description of such syndromes will require understanding of the principles of regional cognitive neurobiology that is not confined to cortical foci but incorporates distributed networks and systems.
From a technical point of view it is possible that the literature surveyed may be biased in several ways. Among case reports, a positive finding with a plausible focal activation is perhaps more likely to be published than a similar study evincing negative findings. Research centres that have access to neuroimaging technologies are more likely to be tertiary centres to which atypical or ‘difficult’ cases are referred. Hence, their findings may have limited application in other settings. Also, this review itself has not addressed all the psychiatric syndromes that might involve bodily disturbance (e.g. anxiety) and the conclusions drawn here must be regarded as provisional.
In summary, many papers implicate association cortices and subcortical brain regions in the generation of abnormal bodily states, but offer limited insight into the specific pathophysiological mechanisms responsible. However, they shed enough light on the phenomenology of the embodied self for us to suggest that doctors should be especially cautious when interpreting physical phenomena that currently cannot be ‘explained’. There are indeed perturbations of brain structure and function associated with many of these ill-understood states, so that our patients’ words offer a first clue as to the systems that may be dysfunctional within their brains. These phenomenological accounts provide a potential signal of the current state of the embodied self.
‘Our flesh is easily worn out; but in being so clearly subject to time and accident it reminds us of what we truly are. Our essence lies in what is most accidental about us – the time and place of our birth, our habits of speech and movement, the flaws and quirks of our bodies’ (Reference GrayGray, 2002: p. 144).
Declaration of interest
None
MCQs
-
1 The following are methods used to image brain function:
-
a structural MRI
-
b computed tomography
-
c functional MRI
-
d SPECT
-
e air encephalography.
-
-
2 The following disorders have been associated with abnormalities of temporo-parietal function:
-
a delusional parasitosis
-
b passivity phenomena
-
c conversion disorder
-
d dysmorphophobia
-
e Cotard's delusion.
-
-
3 The caudate nucleus is:
-
a part of the striatum
-
b a component of the ‘executive’ circuit
-
c implicated in some studies of somatisation disorder
-
d involved in the processing of disgust
-
e implicated in some studies of chronic fatigue syndrome.
-
-
4 The frontal lobes:
-
a lie posterior to the central sulcus
-
b are engaged in planning and programming actions
-
c contain loops projecting to and from the thalamus
-
d are perfectly symmetrical
-
e have centres involved in working memory.
-
-
5 Clinically useful structural data may be obtained from the following:
-
a brain computed tomography
-
b brain MRI
-
c EEG
-
d fMRI
-
e Wada testing.
-
MCQ answers

| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | F | a | T | a | T | a | F | a | T |
| b | F | b | T | b | T | b | T | b | T |
| c | T | c | T | c | T | c | T | c | F |
| d | T | d | T | d | T | d | F | d | F |
| e | F | e | T | e | T | e | T | e | F |







eLetters
No eLetters have been published for this article.