Many of the current drug treatments in psychiatry were discovered without any knowledge of the underlying ‘disease processes’ concerned. Theories of the mechanism of drug action were all developed post hoc and are predominantly concerned with monoamine neurotransmitter modulation. However, not all patients are treated successfully by these means and rates of full remission are frequently quite low. Current diagnostic categorisation of psychiatric disorders, such as unipolar depression, bipolar disorder and schizophrenia, is provisional and subject to ongoing revision. Ideally, drug development should be predicated on knowledge of ‘drug targets’ derived from understanding of the pathophysiological processes involved that are important for the illness in question. Although the central pathophysiological components of most psychiatric disorders remain unknown, a large body of evidence indicates that endocrine dysfunction, particularly of the hypothalamic–pituitary–adrenal (HPA) axis, may be important for certain psychiatric illnesses, as indeed is the case for some physical illnesses. Factors related to the HPA axis may partially account for treatment-resistance (although, of course, a variety of factors contribute) and these may also suggest targets for development of new drugs with novel modes of action.
The hypothalamic–pituitary–adrenal axis
The HPA axis is an endocrine system comprising both central nervous system and peripheral endocrine tissue: the hypothalamus, pituitary and adrenal cortices are its major components. The HPA axis is regulated by external inputs from a number of brain regions, including the amygdala, hippocampus and nuclei within the midbrain. In addition, the HPA axis also contains a number of autoregulatory mechanisms. The paraventricular nucleus of the hypothalamus secretes the peptides corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) into the microportal circulatory system of the pituitary stalk. These peptides have a synergistic effect on the release of adrenocorticotropic hormone (ACTH) from the anterior lobe of the pituitary. Cortisol, a glucocorticoid released from the adrenal cortex in response to ACTH, has a plethora of central and peripheral effects which are mediated primarily via glucocorticoid receptors of types I and II. Under normal homeostatic conditions, type I receptors are mostly saturated, owing to their high affinity for cortisol and other glucocorticoids, and type II glucocorticoid receptors are the more sensitive to stressor-dependant changes within the axis. It is by the glucocorticoid receptor that cortisol primarily exerts its negative feedback on the hippocampus, hypothalamus and pituitary. Under normal conditions, diurnal variation in cortisol levels is seen. In humans, this begins with markedly elevated serum concentrations shortly after awakening, trailing off to the lowest levels in the evening, presenting as a characteristic curve when concentrations are graphed over a 24 h period. Changes in glucocorticoid receptor number or function may be important in altering the homeostatic function of the HPA axis observed in healthy individuals. These regulatory mechanisms are important in determining basal levels and circadian fluctuations in cortisol levels (Reference Dinan and ScottDinan 2005).
HPA abnormalities in psychiatric illnesses
The first observations of abnormal cortisol levels in people with depression were made in England by Board and colleagues (Reference Board, Wadeson and PerskyBoard 1957). Subsequent studies have shown that HPA hyperactivity, as variously manifested by hypersecretion of CRH and AVP, increased cortisol levels in plasma, urine, cerebrospinal fluid and saliva, exaggerated cortisol responses to ACTH, and enlarged hypothalamus, pituitary and adrenal glands, occurs in individuals with a number of severe mood disorders.
In addition to unipolar depression, hypercortisolaemia has since been found in other psychiatric illnesses, including psychotic depression, bipolar disorder, schizophrenia and Alzheimer's disease (Reference Nelson and DavisNelson 1997; Reference Walder, Walker and LewineWalder 2000; Reference Watson, Gallagher and RitchieWatson 2004; Reference DeBattista and BelanoffDeBattista 2005), although these abnormalities in cortisol levels have not always been found in milder depression (using ICD–10 criteria) (Reference CowenCowen 2002). In severe depression, high doses of glucocorticoids have been shown to cause brief elevations in mood, suggesting that cortisol may actually be a resilience factor that the body releases to restore normal serotonergic function (Reference YoungYoung AH 1994a). Hypersecretion of CRH and AVP, causing hypercortisolaemia, may be a result of impaired feedback mechanisms resulting from glucocorticoid receptor abnormalities, such as decreased receptor number or altered function (Reference Dinan and ScottDinan 2005). This view is supported by the demonstration of down-regulated glucocorticoid receptor mRNA in post-mortem frontal and temporal cortices and hippocampi of individuals with unipolar or bipolar disorder or schizophrenia (Reference Webster, Knable and O'GradyWebster 2002).
The cortisol-suppressing activity of the synthetic glucocorticoid dexamethasone is useful as a measure of the functional integrity of the glucocorticoid-receptor-mediated negative feedback mechanism. Reports of cortisol non-suppression in response to dexamethasone in unipolar, bipolar and schizophrenic disorders suggest a primary glucocorticoid receptor abnormality in these disorders (Reference Zhou, Shen and ShuZhou 1987). Post-dexamethasone AVP levels have proven to be a sensitive measure of HPA dysfunction when compared with healthy controls, and levels are elevated in unipolar and bipolar disorder, in both currently symptomatic and remitted patients (Reference Dinan and ScottDinan 2005; Reference Watson, Gallagher and FerrierWatson 2006).
(Key points 1.)
Supplementing the dexamethasone suppression test with subsequent administration of exogenous CRH is known as the dex/CRH test, and recent work utilising this technique has verified that HPA axis function is abnormal in bipolar disorder (Reference Watson, Gallagher and RitchieWatson 2004). This implicates the regulatory mechanisms that involve glucocorticoid receptors and AVP and CRH release in the HPA axis. Response to the dex/CRH test has been found to resolve with successful treatment in acute unipolar depression (Reference Kunugi, Ida and OwashiKunugi 2006), but in bipolar disorder preliminary results show that the abnormality appears stable over time and is independent of mood state, persisting in euthymia (Reference Watson, Gallagher and RitchieWatson 2004).
Cortisol abnormalities in post-traumatic stress disorder
Evidence is conflicting regarding the role of the HPA axis in post-traumatic stress disorder (PTSD). Some studies indicate dysfunction of the axis, showing hypocortisolaemia, rather than hypercortisolaemia, although elevated CRH is still reported (Reference YehudaYehuda 2001). The same group has found evidence indicating that ACTH is also elevated in PTSD; it is therefore likely that the dysfunction in the HPA axis lies in the output of the adrenal glands (Reference YehudaYehuda 2006). This would suggest that antiglucocorticoid treatments may not be able to be used in the same manner for PTSD (because of already low cortisol levels) as for disorders with hypercortisolaemia. Other research groups have found that salivary cortisol levels are actually normal in individuals with PTSD, and that hypercortisolaemia is found only in individuals with PTSD and comorbid major depressive disorder (Reference Young and BreslauYoung EA 2004a).
5-HT and HPA axis interaction
Whether hypercortisolaemia is a contributing risk factor or a resulting effect of the disorders listed above has yet to be determined. However, when considering these putative pathophysiological processes it is important to note that there appears to be complex regulatory interaction between the HPA axis and brain serotonergic systems (for a review see Reference Porter, Gallagher and WatsonPorter 2004).
Studies show that with successful treatment of depression using serotonergic antidepressants (i.e. selective serotonin reuptake inhibitors and serotonin and noradrenaline reuptake inhibitors – SSRIs and SNRIs), non-suppression of the dexamethasone suppression test and other HPA dysfunctions resolve, although this is not fully established for all other antidepressants (Reference Harmer, Bhagwagar and ShelleyHarmer 2003). Precursors of serotonin (5-HT), including L-tryptophan and 5-hydroxytryptophan (5-HTP), have been shown to increase ACTH, CRH and cortisol levels. Some evidence suggests that 5-HT may even act directly on the adrenal glands to release cortisol (for reviews see Reference DinanDinan 1996; Reference Porter, Gallagher and WatsonPorter 2004).
In vivo work on rats has shown that after a bilateral adrenalectomy, 5-HT synthesis is reduced in the hypothalamus, hippocampus and raphe nuclei (Reference DinanDinan 1996). Further animal work on the somatodendritic 5-HT1A autoreceptor indicates that glucocorticoids modulate expression of these receptors in various regions of the brain (Reference Man, Young and McAllister-WilliamsMan 2002). This has been replicated in normal human volunteers by using hydrocortisone to attenuate the effects of the partial serotonin agonist buspirone on the hypothermia and reduced duration of rapid eye movement sleep (Reference Young, Sharpley and CamplingYoung AH 1994b).
The HPA overactivity observed in people with depression appears to alter the diurnal rhythms of the axis, eliminating the normal decrease in cortisol seen in the later part of the day and producing a more sustained release over time. One consequence of this flattened glucocorticoid rhythm may be a reduced clinical efficacy of antidepressant treatments such as chronic SSRIs. Gartside and collaborators modelled this flattened glucocorticoid rhythm in experimental animals and then examined the effects of this manipulation on the neuropharmacological effects of an SSRI (Reference Gartside, Leitch and YoungGartside 2003). They used an implanted corticosterone-releasing pellet (compared with sham pellets) to reproduce the flattened glucocorticoid rhythm and then administered either fluoxetine or vehicle to rodents. In vivo microdialysis was then conducted to quantify effects on extracellular 5-HT levels. With fluoxetine treatment, 5-HT levels were significantly higher in the shampellet group than in the group whose glucocorticoid rhythm had been flattened by corticosterone-releasing pellets. This evidence suggests that elevated glucocorticoids (as found in mood disorders) reduce the ability of SSRIs to elevate extracellular brain 5-HT levels in the forebrain and thus impair the clinical efficacy of these drugs.
Similar findings were observed in a group of fluoxetine-resistant women, who demonstrated HPA-axis overactivity, compared with a group of matched women who were being treated successfully with fluoxetine (Reference Young, Altemus and LopezYoung EA 2004b). Add-on treatment of an antiglucocorticoid drug remedied this non-response and demonstrated HPA axis interference with a conventional SSRI.
Interestingly, in people with remitted depression, the risk of relapse is greatly increased when cortisol levels are chronically increased (Reference Zobel, Nickel and SonntagZobel 2001). Thus, both lack of recovery and a higher risk of relapse may be related to increased cortisol levels, although this could be viewed as a manifestation of unresolved depression. The 5-HT1A receptor number and function have been shown to be reduced in people with depression, and animal studies have shown that 5-HT1A receptor binding is reduced after administration of corticosterone (Reference Mendelson and McEwenMendelson 1992). However, human studies attempting to replicate this possible regulatory mechanism have thus far shown mixed results (for a review see Reference Porter, Gallagher and WatsonPorter 2004).
Consequences of hypercortisolaemia
It is widely recognised that a variety of medicinal steroids can cause neuropsychiatric adverse reactions. A number of case reports from the 1980s revealed that, though rare, even intranasal corticosteroid sprays can trigger relapse into episodes of both mania and psychosis after a period of sustained administration (Reference Lewis and CochraneLewis 1983; Reference Meyboom and De Graaf-BreederveldMeyboom 1988; Reference Goldstein and PreskornGoldstein 1989; Reference PhelanPhelan 1989). Cushing's disease is an endocrine disorder in which hypercortisolaemia occurs, most commonly caused by a tumour in the pituitary but also occasionally by peripheral adrenal lesions. It is now established that cognitive impairments similar to those caused by medicinal steroids are seen across various conditions in which endogenous or exogenous corticosteroids are raised, including both Cushing's disease and severe mood disorders (Reference Wolkowitz, Reus and WeingartnerWolkowitz 1990).
Studies in experimental animals have shown deficits in learning and memory following chronic administration of glucocorticoids (Reference Lupien and McEwenLupien 1997), as well as marked atrophy of neurons in the hippocampal formation. It has been postulated that a similar effect of cortisol may underlie some of the cognitive deficits observed in humans with severe mood disorders (Reference Sapolsky, Krey and McEwenSapolsky 1986).
Clinical data suggest that cortisol treatment induces cognitive deficits in healthy humans, and these deficits appear to be attributed partly to the frontal lobe, suggesting that this brain area may also be sensitive to these effects of cortisol (Reference Young, Sahakian and RobbinsYoung AH 1999). The deficits in healthy volunteers are reversible, but this may not be entirely the case with the cognitive deficits induced by hypercortisolaemia associated with mood disorders if atrophy of tissue is irreversible in long-term illness (Reference Young, Sahakian and RobbinsYoung AH 1999; Reference Thompson, Gallagher and HughesThompson 2005). Indeed, duration of illness is known to be negatively correlated with severity of cognitive deficits (Reference Cavanagh, van Beck and MuirCavanagh 2002). Therefore, early re-establishment of normal HPA activity in mood disorders may be an important therapeutic goal, before irremediable deficits in cognitive function can occur.
Consequences of hypocortisolaemia
Just as excess cortisol has detrimental consequences, so does a deficiency of glucocorticoids. Although elevated cortisol has neurotoxic effects on the above-mentioned regions, cortisol is also known to have a neuroprotective role and it is critical in regulating the innate immune responses of the central nervous system (Reference Glezer and RivestGlezer 2004). In individuals with Addison's disease, primary adrenal failure results in hypocortisolaemia as one of the major presenting traits. Commonly, individuals report a decreased quality of life, due to chronic fatigue and a reduced sense of well-being, and although deficits in cognition are anecdotally acknowledged, they are not yet well-defined (Reference Hunt, Gurnell and HuppertHunt 2000). These tentatively posited consequences of low cortisol levels are important to consider for putative antiglucocorticoid treatments in psychiatry, as therapy of this kind is likely to be most beneficial on a short-term basis.
Suicide risk
When looking at the influence of hypercortisolaemia on risk of suicide, it becomes important to differentiate between suicide attempt and completed suicide. In a number of post-mortem studies, findings have indicated increased measures of HPA axis activity in completed suicide (Reference Dorovini-Zis and ZisDorovini-Zis 1987; Reference Nemeroff, Owens and BissetteNemeroff 1988; Reference Arato, Banki and BissetteArato 1989; Reference Lopez, Palkovits and AratoLopez 1992; Reference Raadsheer, van Heerikhuize and LucassenRaadsheer 1995). Similarly, non-suppression of cortisol has repeatedly been shown to be associated with subsequent completed suicide (Reference LesterLester 1992). From this, one might hypothesise that treatment with antiglucocorticoids might have a specific influence on suicide risk.
This association between HPA axis hyperactivity and subsequent completed suicide seems to be most frequent among severely ill patients (Reference Coryell, Young and CarrollCoryell 2006); it has been found in particular in in-patients with manifested suicide risk. Furthermore, among in-patients with a recent suicide attempt, an association has been demonstrated between non-suppression of cortisol and increased scores on the Suicide Assessment Scale (Reference Westrin and NimeusWestrin 2003). However, attempted suicide seems to be associated not with HPA axis hyperactivity (Reference LesterLester 1992) but rather with hypoactivity of the HPA axis (Reference Pfennig, Kunzel and KernPfennig 2005). Consequently, when evaluating the effects of antiglucocorticoids in clinical practice and in future research, a thorough assessment of suicide risk, as well as suicidal behaviour, should be taken into consideration.
Therapeutic targets
There is increasing evidence to suggest that the consequences of HPA dysfunction described above are central to the pathogenesis of severe affective disorders and cognitive deficits (Reference McQuade and YoungMcQuade 2000). Modulation of the effects of hypercortisolaemia may provide potential treatments for mood disorders, and such strategies are the focus of considerable research interest.
Dehydroepiandrosterone
The adrenal steroid dehydroepiandrosterone (DHEA) has been used with some success in the treatment of depression (Reference Wolkowitz, Reus and KeeblerWolkowitz 1999). The physiological functions of DHEA, and its sulphated conjugate DHEA–S, are numerous and it is now known to act as both an active hormone and a prohormone for sex steroids. It may have antidepressant qualities and it is speculated that its therapeutic effects may be accounted for by its potentially antiglucocorticoid properties (Reference Wolkowitz, Reus and KeeblerWolkowitz 1999).
One proposed mechanism for the anti-glucocorticoid effects of DHEA is that the molecule aids in reversing nuclear localisation of the glucocorticoid receptor caused by its interaction with cortisol (Reference Cardounel, Regelson and KalimiCardounel 1999). Another explanation is that DHEA is partially metabolised to testosterone and oestrogen, both of which have effects on mood. Other work, in mice, shows that the hormone has dose-dependant neuroprotective effects in the hippocampus and suggests that this quality is attributed to the hormone directly, independently of its subsequent products (Reference Cardounel, Regelson and KalimiCardounel 1999).
In a study of the molar ratio between cortisol and DHEA in drug-free patients with depression, we (A.Y. and colleagues) found reduced DHEA levels and a correspondingly elevated cortisol: DHEA ratio. This proved to be a more sensitive measure in depression than basal cortisol alone (Reference Young, Gallagher and PorterYoung AH 2002). It is well established now that age-related declines in DHEA levels possibly reduce the effectiveness of the body's ‘natural’ anti-glucocorticoid mechanisms.
Steroid synthesis inhibitors
High cortisol levels can be lowered by inhibiting steroid synthesis from cholesterol. Ketoconazole, metyrapone and aminoglutethimide are three commonly employed compounds, each of which acts to inhibit multiple enzymes involved in the creation of cortisol from cholesterol. Early studies of their use as antidepressant therapies reported promising results, showing successful reduction of ACTH and cortisol levels, correlated with improvements in mood (Reference MurphyMurphy 1997). Ketoconazole, when administered daily, reduced both cortisol levels and depressive symptoms within 72 h in an individual with treatment-resistant depression (Reference Ravaris, Sateia and BerozaRavaris 1988). Another study examined whether the addition of metyrapone to standard serotonergic antidepressants induced a more rapid, efficacious and sustained treatment response in patients with major depression (Reference Jahn, Schick and KieferJahn 2004). Of 63 individuals with major depression and taking nefazodone or fluvoxamine, 33 were chosen at random also to receive metyrapone for 3 weeks of a 5-week trial. Metyrapone was reported to be an effective adjunct, accelerating the onset of antidepressant action. Serum antidepressant levels did not differ between the groups, thus suggesting that this acceleration was not due to a peripheral pharmacokinetic effect. Both a better treatment outcome and a greater antidepressive effect, sustained for the period of observation, were noted in the group receiving metyrapone augmentation.
Statins
Cholesterol is the basic building block of steroid hormones, and although they do not act directly on hormones, cholesterol synthesis inhibitors (‘statins’) have been studied as potential means of reducing available adrenal steroids. It is of note that statins have been reported to be associated with an increased incidence of behavioural and personality disorders that may be hormonally influenced (Reference Huffman and SternHuffman 2007).
The use of atorvastatin and lovastatin has been linked to an increase in dopamine and homovanillic acid (HVA) levels (Reference Ormiston, Wolkowitz and ReusOrmiston 2004), but no significant effect has yet been shown on steroid hormones or serotonin. Moreover, lowering of cholesterol may actually be attributed to an increase in depressive symptoms. In a study of 20 patients with hypercholesterolaemia treated with a variety of drugs, cholesterol was effectively reduced whereas the number of depressive symptoms significantly increased, although remaining at subclinical levels in all participants (Reference Delva, Matthews and CowenDelva 1996).
Corticotropin-releasing hormone antagonists
Oversecretion of CRH, resulting in hypercortisolaemia, may be normalised by acute blockade of CRH receptors in the pituitary by means of compounds such as antalarmin and astressin, the withdrawal of which causes an increased signal in the normal HPA feedback loop. These drugs are still in preclinical stages but preliminary results suggest that these CRH antagonists indeed have clinical potential (Reference Broadbear, Winger and RivierBroadbear 2004; Reference French, Fite and JensenFrench 2007). Other drug development programmes are targeting central CRH receptors that are further from the HPA axis. We await the results of ongoing clinical investigations.
Glucocorticoid receptor agonists
Activation of the glucocorticoid-receptor-mediated negative-feedback mechanism that regulates cortisol levels is another strategy for reducing circulating cortisol levels. The synthetic glucocorticoid dexamethasone given at doses of 3–4 mg for 4 days has putative antidepressant effects (Reference Arana, Santos and LaraiaArana 1995). At this dose, dexamethasone does not enter the central nervous system and consequently central glucocorticoid receptors are not activated (Reference Karssen, Meijer and BerryKarssen 2005). However, glucocorticoid receptors at the level of the pituitary are activated, leading to a lowering of endogenous circulating cortisol. The brief course of dexamethasone administration in these studies avoids the side-effects associated with longer-term treatment.
Glucocorticoid receptor antagonists
Glucocorticoid receptor antagonists have also been advocated as agents with potential therapeutic properties for mood disorders. This is based on the ability of the glucocorticoid receptor antagonist to block any detrimental effect of hypercortisolaemia and on the theoretical ability of an antagonist to up-regulate its receptor. Administration of a glucocorticoid receptor antagonist results in an acute antiglucocorticoid effect, while presumably causing a compensatory up-regulation of glucocorticoid receptor numbers, leading to enhanced negative feedback on the HPA axis after the antagonist has been discontinued. Initial clinical studies using the antagonist RU-486 (mifepristone) have shown some positive results, but some clinical efficacy may have been masked by the prolonged administration of the drug (Reference Murphy, Filipini and GhadirianMurphy 1993). Animal studies suggest that glucocorticoid receptor numbers are increased rapidly (within hours) after the administration of RU-486, which may restore normal feedback, thus ‘resetting’ the HPA axis. Such data suggest that a brief period of treatment with the antagonist may be adequate for restoring normal HPA axis function.
RU-486 has been evaluated both as augmentation and as monotherapy in the treatment of psychotic major depression. In a group of in-patients with the disorder, an open-design, open-label study involving acute augmentation (7 days) of current medications with 600 or 1200 mg RU-486 daily produced significant reductions in scores on the Brief Psychiatric Rating Scale and in measures of depressive symptoms compared with baseline, although patients who received only 50 mg RU-486 daily showed little or no benefit (Reference Belanoff, Rothschild and CassidyBelanoff 2002). Similar findings were replicated in a study in which a group of patients taking neither conventional antidepressant nor antipsychotic medications received 7 days of RU-486 treatment for psychotic major depression (Reference DeBattista and BelanoffDeBattista 2005).
One of us (A.Y.) has been involved in a double-blind cross-over pilot study of RU-486 for treatment-resistant bipolar disorder (Reference Young, Gallagher and WatsonYoung AH 2004). After baseline data were collected, the 20 participants received either placebo or 600 mg/day RU-486 for 7 days, followed by 14 days of wash-out, at which point the cross-over began. During the study, neurocognitive and neuroendocrine function and mood symptoms were measured weekly. Following treatment with RU-486, a selective improvement in neurocognitive functioning and significant improvements in mood symptoms were observed, compared with placebo. The improvement in cognition was inversely correlated with basal cortisol levels, adding to the plausibility that this was an antiglucocorticoid effect. These data require replication but provide preliminary evidence that glucocorticoid antagonists may be effective in the treatment of cognitive deficits and mood symptoms associated with bipolar disorder. Interestingly, a similar protocol in patients with schizophrenia suggested no beneficial effect on symptoms or cognitive impairments, possibly because these patients had little evidence of a dysfunctional HPA axis (Reference Gallagher, Watson and SmithGallagher 2005).
(Key points 2.)
Conclusions
Our understanding of the biological origins of psychiatric illnesses is beginning to expand beyond the narrow realm of monoamine neurotransmitter function, and the past decade has seen an increased understanding of how the hypothalamic–pituitary–adrenal (HPA) axis is involved in a number of major disorders. One of the most common expressions of HPA axis dysfunction is an excess of the glucocorticoid cortisol. Hypercortisolaemia may impair the effectiveness of current medications and has been associated with a characteristic pattern of neurocognitive deficits. High levels of this hormone may also correlate with a greatly increased rate of suicide. Different medications have been used in clinical trials to counteract hyperactivity of the HPA axis and restore normality, and the effects on both depressive symptoms and neurocognition have been positive. Recent data suggest that glucocorticoid receptor agonists, antagonists and steroid synthesis inhibitors may be useful in the treatment of mood disorders (Reference Gallagher, Malik and NewhamGallagher 2008), although more definitive large-scale clinical trials will be required to fully establish efficacy of this treatment strategy.
MCQs
-
1 Cortisol is responsible for negative feedback in the HPA axis, and mainly by acting on:
-
a the hippocampus alone
-
b the hypothalamus alone
-
c the pituitary alone
-
d the hippocampus and the pituitary
-
e the hippocampus, the hypothalamus and the pituitary.
-
-
2 Abnormalities of the HPA axis that may be common in mood disorders include:
-
a glucocorticoid receptor function
-
b increased AVP levels
-
c decreased CRH levels
-
d volume of the pituitary gland
-
e adrenal sensitivity to ACTH.
-
-
3 Clinical trials suggest that symptoms of depression may be improved by:
-
a CRH agonists
-
b antalarmin
-
c astressin
-
d atorvastatin
-
e RU-486.
-
-
4 Glucocorticoid receptor antagonists are thought to exert their therapeutic effect by:
-
a up-regulating glucocorticoid receptor receptors
-
b down-regulating glucocorticoid receptor receptors
-
c blocking conversion of DHEA to cortisol
-
d blocking the effects of ACTH
-
e blocking the effects of CRH and AVP.
-
-
5 A factor increasing risk for dying by suicide may be:
-
a female gender
-
b hypocortisolaemia
-
c hypercortisolaemia
-
d dexamethasone hypersensitivity
-
e age between 25–35 years.
-
MCQ answers
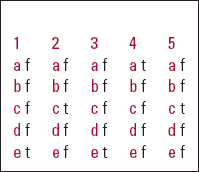
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | f | a | f | a | f | a | t | a | f |
| b | f | b | f | b | f | b | f | b | f |
| c | f | c | t | c | f | c | f | c | t |
| d | f | d | f | d | f | d | f | d | f |
| e | t | e | f | e | t | e | f | e | f |

|

The following may be useful in the treatment of mood disorders:
|







eLetters
No eLetters have been published for this article.