There are major gaps in our understanding of the mode of action of antidepressant treatments (Reference Racagni and PopoliRacagni 2008). This is the case for the anti-depressant drugs that are in everyday use as much as for electroconvulsive therapy (ECT). Gaps in knowledge are not, however, synonymous with ignorance. The presumption of a state of ignorance about the mode of action of ECT risks the neglect of what is known. The aims of this article are to remind readers of some of the seminal studies that have been conducted into the mode of action of ECT and to explain how original theories have been refined by research over the past 20 years. I will concentrate on research in humans, much of which has been facilitated by new techniques of brain imaging. I have not given references for research studies that had negative findings, but they are available from me.
The laboratory and clinical research into the mode of action of ECT before the era of safe techniques to study human brain function were well covered in the monograph by Reference Malitz and SackeimMalitz & Sackeim (1986). Neuroscientists still undertake a great deal of laboratory work with the animal model of ECT to try to identify mechanisms of relevance to antidepressant treatments in general. They do so because they recognise ECT as the most efficacious treatment for major depression. Electroconvulsive therapy is now used predominantly in the treatment of depressive illness, and this indication will be the main, but not exclusive, topic of this article.
The importance of the generalised cerebral seizure
Seminal studies
Almost 30 years ago, an ex-President of the Royal College of Psychiatrists, the late Professor Bob Kendell, reviewed the existing studies on the mode of action of ECT (Reference KendellKendell 1981). He concluded that there was ‘compelling evidence that the induction of generalized seizure activity in the brain was the crucial [therapeutic] element’. His review was widely quoted and his conclusion was the accepted theory for most of that decade.
In his consideration of the efficacy of ECT for depressive illness, Kendell commented that ECT was in widespread use before the randomised controlled trial was introduced into clinical medicine. It is therefore possible to make a number of methodological criticisms of early work in comparison to the standard of evidence required today. The study of the mode of action of ECT would be pointless if it were not an effective treatment for depressive illness. The UK ECT Review Group used modern methods to evaluate the evidence on the efficacy of ECT for depressive disorders. In particular, the group conducted a systematic review and meta-analysis; the efficacy of ECT as a short-term treatment for major depression was clearly confirmed (The UK ECT Review Group 2003). The available evidence also suggested that ECT was more efficacious than drug treatment.
The challenge to seminal studies
A study conducted at the New York State Psychiatric Institute (NYSPI) was a serious challenge to what Kendell had referred to as compelling evidence that the induction of generalised seizure activity was the crucial therapeutic element in ECT (Reference Malitz and SackeimMalitz 1986). The NYSPI study was a randomised controlled trial of ECT in the treatment of primary major depression. There was one group of patients in whom only 28% responded to ECT, no more than that seen in patients treated by simulated ECT. This low rate of response occurred despite the fact that the induced generalised convulsions lasted an average of 44 s, and the occurrence of cerebral seizure activity was confirmed by monitoring with electroencephalogram (EEG). This group of patients had been treated with right unilateral ECT and this electrode placement was combined with electrical stimulation that was only just sufficient to induce generalised cerebral seizure activity. This became known as threshold electrical stimulation. The authors concluded that the classical theory was wrong: the generalised cerebral seizure may be a necessary therapeutic ingredient, but it was not sufficient on its own.
The reconciliation of apparently divergent findings
Xenon inhalation technique
Researchers at the NYSPI tried to understand why threshold right unilateral ECT lacked any antidepressant effect by investigating its acute effects on regional cerebral blood flow (rCBF). They measured rCBF with the xenon inhalation technique conducted 25 min before and 50 min after an individual ECT session (Reference Malitz and SackeimMalitz 1986). The findings with threshold right unilateral ECT were then compared with those of threshold bilateral ECT.
In general, ECT led to a reduction in blood flow, which was particularly marked over the anterior cortex. The more striking finding was that the reduction was symmetrical after bilateral ECT, but restricted to the right hemisphere after threshold right unilateral ECT. There was a suggestion of an interaction among the reduction in cerebral blood flow, electrode placement and likelihood of clinical remission. The researchers concluded that these preliminary findings merited further investigation.
Electrical dose
There also followed a series of treatment studies in patients with depression at NYSPI to investigate how manipulations of the electrical stimulus affected the clinical efficacy of ECT (Reference Sackeim, Prudic and DevanandSackeim 2000). It turned out that the extent to which the electrical dose exceeded the seizure threshold was critical in determining the clinical efficacy of unilateral ECT. The eventual conclusion was that when the electrical dose in right unilateral ECT exceeded the seizure threshold by 500% (i.e. six times the seizure threshold), then it was as efficacious as bilateral ECT.
The conclusion of the equivalence of efficacy between high-dose right unilateral placement and bilateral placement has been debated. The number of study patients was inadequate to have the statistical power to conclude confidently that high-dose right unilateral ECT was equivalent in efficacy to bilateral ECT. A recently completed randomised controlled trial, which included the largest number of patients to date, did not support the conclusion that high-dose right unilateral ECT was as efficacious as bilateral ECT, with an electrical stimulation 50% above the seizure threshold (Reference Kellner, Knapp and HusainKellner 2010). It is also important to note that the NYSPI treatment studies included an option for patients to cross over from right unilateral treatment to bilateral treatment in the case of inadequate clinical response to right unilateral ECT. Most patients who needed to cross over did recover with bilateral ECT and this included patients who had not responded to high-dose right unilateral ECT (Reference Sackeim, Prudic and DevanandSackeim 2000).
Single photon emission computed tomography
The xenon inhalation technique is cumbersome, lacks spatial resolution and can only measure blood flow in the cerebral cortex. In contrast, single photon emission computed tomography (SPECT) is particularly well suited to the investigation of rCBF during seizures. A collaboration among neurologists and psychiatrists at Yale University School of Medicine suggested hypotheses to explain both the reduced clinical efficacy of right unilateral ECT and the reduced effect on verbal memory (Reference Enev, McNally and VargheseEnev 2007). It would have been informative to compare threshold right unilateral ECT with threshold bilateral ECT, but threshold right unilateral ECT was not included. Presumably, this would have been regarded as unethical because threshold right unilateral ECT had already been shown to be no more efficacious than simulated ECT. The researchers therefore only included a type of right unilateral ECT expected to have greater efficacy than threshold right unilateral ECT, that is, with an electrical dose 2.5 times the measured seizure threshold.
The findings challenged some of the accepted ideas about generalised cerebral seizures (Box 1). It turned out that how the seizure is induced has a substantial bearing on its generalisation. In particular, cerebral seizures induced by right unilateral ECT were asymmetrical not only in the cortex, but also in subcortical regions; there was little change in blood flow in the left medical thalamus and left temporal lobe. Bilateral ECT produced robust increases of blood flow symmetrically in frontal and temporal regions of the brain. In right unilateral ECT, the relative sparing of the left temporal lobe was the likely explanation of the reduced impairment of verbal memory, and the relative sparing of the left cortical and subcortical regions was the likely explanation of the reduced clinical efficacy (Reference Enev, McNally and VargheseEnev 2007). More will be said about the putative neuroanatomy of major depression below.
BOX 1 ECT-induced cerebral seizures: insights from brain imaging
-
• Cerebral seizures are not truly generalised, even if associated with spike–wave rhythms on EEG
-
• Cerebral seizures:
-
propagate from the site of initiation
-
involve specific regions of the brain and spare others
-
differ between right unilateral and bilateral ECT
-
are asymmetrical in right unilateral ECT, with relative sparing of left cortical and subcortical regions
-
Cerebral seizures and the neuroanatomy of major depression
The accepted view of so-called generalised cerebral seizures is that only specific networks of neurons are selectively involved and that specific nodes within these networks are crucial for the propagation and manifestations of such seizures. These evolving ideas about cerebral seizures have striking similarities to evolving ideas about the neuroanatomy of major depression (Reference MaybergMayberg 2003).
One such so-called node in the putative neuroanatomy of depression is the subgenual anterior cingulate cortex. The cingulate cortex is one of the largest parts of the limbic system. It wraps itself around the corpus callosum like a belt, which gives it its name. Its anterior part plays a crucial role in goal-directed behaviours in terms of initiation and motivation. A part of the anterior cingulate cortex lies ventral to the genu of the corpus callosum; hence, it is referred to as the subgenual anterior cingulate cortex. Brain imaging studies have suggested that this region is involved in the modulation of negative mood states; the cortex on the left cerebral hemisphere has been particularly implicated with depressed mood. This region is now one of the target sites for the treatment of major depression with deep brain stimulation. Reference Mayberg, Lozano and VoonMayberg et al (2005) reported that chronic electrical stimulation of white matter tracts adjacent to this region was associated with sustained remission from treatment-resistant major depression in four of six patients.
One recent ECT study merits particular mention in the context of these evolving ideas. Positron emission tomography (PET) was used to assess glucose metabolism immediately before and 2–3 weeks after a course of either unilateral or bilateral ECT in ten patients with major depression and psychotic features (Reference McCormick, Ponto and PiersonMcCormick 2007). The antidepressant effect of a course of ECT, measured as change on the Hamilton Rating Scale for Depression, was significantly correlated with increased metabolism in the left subgenual anterior cingulate cortex and the hippocampus. Clearly, such a small study must be regarded as preliminary. Nevertheless, it suggested that repeated cerebral seizures have specific effects on neuronal networks implicated in the putative neuroanatomy of depressive illness. The hippocampus has not previously been identified as a node of potential relevance to the mode of action of ECT, but it may be that its function is affected by the extent of generalisation of cerebral seizure activity.
Practical implications
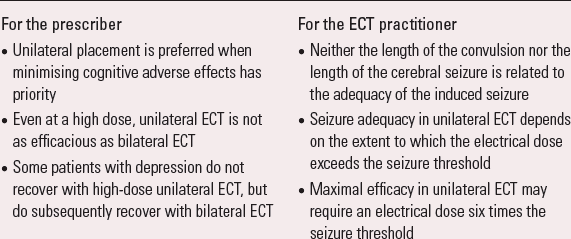
These findings have implications both for the prescription of ECT and its administration (Box 2). Perhaps the key finding for the prescriber of ECT is that there are patients with depression who do not recover with high-dose right unilateral ECT, but do subsequently recover with bilateral ECT (Reference Sackeim, Prudic and DevanandSackeim 2000).
BOX 2 Practical implications of findings on the mode of action of ECT
For the prescriber
|
For the ECT practitioner
|
It will not surprise the ECT practitioner that the length of the convulsion is not related to the adequacy of the induced cerebral seizure: one aim of anaesthesia is to reduce the convulsive muscular activity by the administration of a muscle relaxant drug, which will disassociate the cerebral seizure activity and the convulsion.
It is disappointing that the EEG is not more useful. Although EEG monitoring can be valuable in confirming the induction of cerebral seizure activity, once induced it cannot confirm adequacy in the individual patient. It should be acknowledged that early research used only single-channel EEG monitoring and only length as a measure of adequacy. Subsequent work at NYSPI established that this inability to define adequate cerebral seizures also pertains when more complex measures are made with multichannel recording (Reference Perera, Luber and NoblerPerera 2004).
The key finding for the ECT practitioner is the relationship between cerebral seizure adequacy and the extent to which the electrical dose exceeds the seizure threshold; this is particularly important in unilateral ECT. Advice about the appropriate electrical dose for individual patients is given in guidance produced by the Royal College of Psychiatrists (Reference ScottScott 2005).
Neurochemical effects of repeated seizures
Kendell also considered the pharmacology of repeated seizures in laboratory experiments on animals. He concluded that this work demonstrated behavioural changes that were produced by repeated convulsions induced in similar ways and over a comparable time to ECT in humans. Moreover, the pharmacological characteristics of these behavioural changes suggested that they were produced by changes in central neurotransmission similar to those produced by antidepressant drugs. In particular, Kendell noted increases of functional measures in neuronal systems that used dopamine, noradrenaline and serotonin as neurotransmitters. A later review can be recommended because it includes more laboratory evidence on this topic and also covers reproducible actions on brain neurotransmitters other than monoamines (Reference Nutt, Glue and CoffeyNutt 1992).
Kendell did comment that these experiments had been carried out in animals rather than in individuals with depression, a comment that remains germane. He was concerned that these effects in laboratory animals were temporary, lasting only days rather than weeks after treatment. It turned out that the antidepressant effect of ECT in patients is also temporary: even after a successful course of ECT, relapse within 1 week of the last ECT is a recognised clinical problem if patients remain medically untreated (Reference Rasmussen, Muller and KnappRasmussen 2009). This observation supports the relevance of the laboratory findings.
The monoamine hypothesis
Dopamine and noradrenaline are catecholamines and serotonin is an indoleamine, but together these neurotransmitters are usually known as the monoamines. It is assumed that the reader will be familiar with the monoamine hypothesis of the mode of action of antidepressant drugs (Reference Racagni and PopoliRacagni 2008). The evidence cited by Kendell could simply be used to argue that ECT be regarded as an antidepressant treatment with a triple action in terms of the monoamine hypothesis. Certainly, the hypothesis helps explain some of the specific clinical effects of ECT. For example, a robust finding from laboratory experiments in animals is that cerebral seizures have early effects on the function of both dopamine and noradrenaline, indeed a single seizure has been shown to increase functional measures of dopamine (Reference Nutt, Glue and CoffeyNutt 1992). Reference Nutt, Glue and CoffeyNutt & Glue (1992) commented that the laboratory findings have resonance with the clinical observation of patients with depression treated with ECT. Clinical lore was that psychomotor retardation was a sign of depressive illness that responded early during a course of ECT, and that patients with severe depression may temporarily accept food or water after only one ECT. In fact, a small study by Reference Browning and CowenBrowning & Cowen (1986) did confirm that both psychomotor retardation and impaired appetite are features of depressive illness that start to improve early in a course of ECT. Dopamine function is important in both locomotion and feeding. Noradrenaline function contributes to alertness and energy. An increase in the function of neuronal systems that use dopamine and noradrenaline is an entirely plausible explanation for these clinical observations. Increased dopamine function may also explain the efficacy of ECT in indications beyond major depression, for example, neuropsychiatric disorders such as Parkinson's disease (Reference ScottScott 2005).
There has also been direct evidence of increased dopamine function in patients treated with ECT. For example, cerebrospinal fluid was obtained by lumbar puncture before and after eight unilateral ECT sessions in six patients with depression who had not taken any psychotropic drugs for 14 days before treatment; the concentration of homovanillic acid, the major metabolite of dopamine, increased significantly and a rise in concentration was seen in all six patients (Reference Nikisch and MathéNikisch 2008).
One of the latest brain imaging studies used PET to investigate the effect of a course of bilateral ECT on dopamine D2 receptors in seven patients with major depression, all of whom showed subsequent clinical improvement (Reference Saijo, Takano and SuharaSaijo 2010). There was a 25% fall in D2 receptor binding in an area of the right anterior cingulate cortex rostral to the genu of the corpus callosum. A plausible explanation for the fall was that it was an adaptation to a higher concentration of dopamine in this brain region. Once again, a small study must be regarded as preliminary, but it is interesting that this finding was obtained in the anterior cingulate cortex, an area crucial to goal-directed behaviours.
It may seem counterintuitive to some readers that ECT increases dopamine function. Electroconvulsive therapy is particularly efficacious in major depression with psychotic features. In other indications, antipsychotic effects suggest instead antidopaminergic effects as in the case of antipsychotic drugs. In fact, several strands of evidence suggest that severe depression is associated with reduced dopamine function (Reference Dunlop and NemeroffDunlop 2007). Unlike ECT, most treatments for depression do not directly increase dopamine function.
Beyond the monoamine hypothesis
There are clinical observations and research findings in patients with depression that suggest a need to go beyond the monoamine hypothesis to explain fully the mode of action of ECT (Box 3). Perhaps the most important observation for prescribers is that a failure of patients with depression to recover with a selective serotonin reuptake inhibitor or a monoamine oxidase inhibitor has no bearing on the probability of remission with subsequent ECT. In contrast, a failure to recover with a tricyclic antidepressant drug or bupropion reduces the probability of recovery with subsequent ECT; nevertheless, a half of such patients will recover with ECT (Reference Dombrovski, Mulsant and HasketDombrovski 2005).
BOX 3 Evidence for the need to go beyond the monoamine hypothesis to fully explain the mode of action of ECT in patients with depression
-
• In unipolar major depression:
-
• failure to recover with a selective serotonin reuptake inhibitor or a monoamine oxidase inhibitor has no bearing on the probability of remission with subsequent ECT
-
• half of patients who have already failed to recover with a tricyclic antidepressant drug will experience remission with subsequent ECT
-
• Serotonin transporter gene allelic status has no bearing on treatment outcome with ECT in unipolar or bipolar major depression
-
• Neither acute tryptophan depletion (which in turn depletes serotonin), nor acute catecholamine depletion, nor the two combined causes re-emergence of symptoms after successful treatment of major depression with ECT
These findings have proved controversial and some further comment is merited. First, these findings are relevant only to patients with unipolar, non-psychotic major depression. Studies that included other types of depressive illness, such as delusional depression and bipolar depression, do not find that medication resistance affects the likelihood of remission with ECT (Reference Husain, Kevan and LinnellHusain 2004). Second, there have been later studies that have failed to replicate these findings, but they have either been of a small scale or the number of included patients with a history of medication resistance was small; in either case, they have lacked statistical power. The controversy concerns only the extent to which medication resistance influences the probability of recovery with ECT. There is no doubt that ECT can bring about recovery when drug treatment has not.
Evolving theories on the mode of action of antidepressant drugs
One major and unsolved conundrum about the monoamine hypothesis is that physical treatments for depressive illnesses have immediate effects on monoamines, and yet remission or recovery, if it happens at all, takes several weeks. There have been several new lines of enquiry to identify the biological processes that follow the immediate effects on monoamine function and that are associated with remission from major depression.
Neurogenesis
The birth of neurons, or neurogenesis, is not restricted to the prenatal or early postnatal period. Neurogenesis occurs in the adult mammalian brain (Reference Balu and LuckiBalu 2009). Various stressors reduce neurogenesis in the hippocampus of laboratory animals. In contrast, effective antidepressant treatments promote neurogenesis in laboratory animals; this phenomenon is particularly marked with the animal model of ECT and has been demonstrated in non-human primates (Reference Perera, Coplan and LisanbyPerera 2007). The molecular and cellular theory of depression had already suggested that the antidepressant effect of treatments resulted from evolving intracellular mechanisms that increased neurotropic factors necessary for the survival and function of particular neurons (Reference Duman, Heniger and NestlerDuman 1997). Interest in neurogenesis increased with the confirmation in 1998 that new neurons could be created in the adult brain of humans (Reference Eriksson, Perfilieva and Björk-ErikksonEriksson 1998). However, neurogenesis in the human brain is not as robust as it is in laboratory animals and its physiological significance for the working of the human brain is not yet known. The latest laboratory experiments suggest that the behavioural effects of antidepressant treatments can be disassociated from their effect on neurogenesis. It takes time for antidepressant treatments to promote neurogenesis and this may suggest a link to the time taken for the full antidepressant effect of treatment to evolve. No evidence for such a link has yet been found in patients with depression.
Gene transcription
Remission from depression may be associated with the gradual modification of gene expression by antidepressant treatments. The direct measurement of the products of gene transcription cannot be undertaken in the brain of living humans. But gene polymorphisms can be identified in patients with depression and are of interest because of their possible association with vulnerability to major depression and also treatment response (Reference Rot, Matheu and CharneyRot 2009).
In a Finnish cohort of patients with treatment-resistant major depression, two polymorphisms associated with dopamine metabolism affected the likelihood of remission with ECT (Reference Huuhka, Anttila and HuuhkaHuuhka 2008). The greatest probability of remission after a course of ECT was seen in patients with polymorphisms believed to lead to the lowest concentrations of dopamine in the prefrontal cortex of the brain. Clearly, this will require replication in an independent sample. Nevertheless, it is another strand of evidence that supports the involvement of catecholamine metabolism in the mode of action of ECT.
Brain-derived neurotrophic factor
There is one product of gene transcription that can be measured in the blood of patients which is relevant to the molecular and cellular theory of the antidepressant action mentioned earlier: brain-derived neurotrophic factor (BDNF). This is a protein encoded by its own gene. It acts on the central nervous system to support survival of existing neurons and encourages the growth and differentiation of new neurons. The restoration of ordinary concentrations of this growth factor may be one mechanism of the longer-term effects of antidepressant treatments (Reference Racagni and PopoliRacagni 2008). A preliminary report said that a course of ECT led to an increase in the serum concentration of BDNF; when the full report was published, it became clear that serum BDNF concentration did not in fact change until after the course of ECT (Reference Bocchio-Chiavetto, Zanardini and BortolomasiBocchio-Chiavetto 2006).
There have been several further studies of how a course of ECT affects BDNF, but the findings have been inconsistent. It remains to be established that an increase in the production of growth factors is relevant to the mode of action of ECT, but the suggestion merits further research.
Neurochemical effects beyond monoamines
The most fruitful line of enquiry recently has been the use of modern brain imaging techniques to study neurochemical changes that occur over a course of ECT and that are associated with remission. One of the most important excitatory neurotransmitters in the brain is glutamate and one of the most important inhibitory neurotransmitters is γ-aminobutyric acid (GABA) (Reference Rot, Matheu and CharneyRot 2009). The concentration of both these neurotransmitters can be measured in some regions of the brain by proton magnetic resonance spectroscopy (1H-MRS).
The concentration of GABA in the occipital cortex almost doubled after a course of ECT in eight patients with major depression; an increase was seen in seven out of the eight patients (Reference Sanacora, Masson and RothmanSanacora 2003). There was no statistically significant correlation between improvement in depressive symptoms over the course of treatment and the observed change in cortical GABA concentration, although this small study may have lacked statistical power. A subsequent study found that cortical GABA concentration did not increase after recovery from major depression brought about by cognitive–behavioural psychotherapy. This made it more likely that the observed effect on GABA was a treatment effect of ECT.
A pilot study with SPECT provided further evidence that remission with a course of ECT is associated with enhanced GABA function (Reference Mervaala, Könönen and FöhrMervaala 2001). Single photon emission computed tomography was used to measure not rCBF but the uptake of iomazenil. This ligand binds specifically to the benzodiazepine receptor, which in turn is coupled to the GABA receptor. All five patients with severe depression experienced remission with a course of bilateral ECT and this was associated with widespread increased uptake of iomazenil in the cerebral cortex.
Proton magnetic resonance spectroscopy was also used to study the concentration of glutamate in the anterior cingula in 17 patients with severe unipolar depression treated with ECT (Reference Pfleiderer, Michael and ErfurthPfleiderer 2003). The concentration of glutamate was lower than that observed in individuals without depression before ECT, and increased after the course of treatment in those patients whose depression remitted. This increase was not observed in the patients who did not respond to ECT. These findings were compatible with an earlier study where the concentration of glutamate was measured in the left dorsolateral prefrontal cortex and treatment was given with a right unilateral electrode placement (Reference Michael, Erfurth and OhrmannMichael 2003).
These findings were important for several reasons. First, the studies included patients with depression treated with ECT. Second, there is accumulating evidence that alterations in the function of GABA and glutamate are associated with untreated depressive illness. Third, these findings may be of relevance beyond the antidepressant effect of ECT. It is efficacious in mania as well as major depression and therefore may be better regarded as a mood stabiliser rather than solely as an antidepressant treatment (Reference Malitz and SackeimMalitz 1986). An ECT-induced change in inhibitory neurotransmission could be just as relevant for hypotheses about its mode of action in mania, depending on the specific area of the brain involved. Fourth, these findings suggest testable hypotheses for future research. It is possible to use 1H-MRS to measure both glutamate and GABA simultaneously in patients with depression. Proton magnetic resonance spectroscopy could be conducted before and at the end of a course of ECT and any change in excitatory and inhibitory neurotransmission, and the balance between the two, compared with the extent of the antidepressant effect over the course of treatment. A course of ECT may increase cortical inhibition, which in turn may be a reflection of the balance among neuronal pathways using GABA, glutamate and dopamine as neurotransmitters (Reference Bajbouj, Lang and NiehausBajbouj 2006). Any change in cortical inhibition could also be compared with any change in neurotransmission.
Conclusions
A number of the early brain imaging studies in ECT (e.g. Reference Scott, Turnbull and BlaneScott 1991) rather defensively considered the possibility that the treatment led to some kind of damage to the brain. No such evidence was found. Now modern brain imaging techniques are used more productively to study the mode of action of ECT in living patients with major depression. Generalised cerebral seizure activity is the crucial therapeutic element. It is now known that the choice of electrode placement has a large effect on the generalisation of the induced seizure. Bilateral ECT induces a more widespread and symmetrical seizure. The latest studies concern neurochemical changes that occur over a course of ECT and that are associated with remission. There are preliminary relevant findings of increased concentrations of the neurotransmitters dopamine, GABA and glutamate in certain areas of the brain. Future studies have the potential to replicate these findings and also to set them in the context of the putative neuroanatomy and neuropathology of major depression (Reference Rot, Matheu and CharneyRot 2009).
MCQs
Select the single best option for each question stem
-
1 The probability of remission from unipolar major depression with treatment by ECT is reduced if the patient has already failed to recover with a:
-
a monoamine oxidase inhibitor
-
b selective serotonin reuptake inhibitor
-
c tricyclic antidepressant
-
d none of the above
-
e all of the above.
-
-
2 Which one of the following neurotransmitters is not implicated in the mode of action of ECT:
-
a acetylcholine
-
b dopamine
-
c GABA
-
d glutamate
-
e noradrenaline.
-
-
3 Which one of the following neurotransmitters is implicated in ECT's beneficial effect on psychomotor retardation:
-
a acetylcholine
-
b dopamine
-
c GABA
-
d glutamate
-
e noradrenaline.
-
-
4 Bilateral ECT has greater efficacy than unilateral ECT in the treatment of major depression. The most plausible reason is that in bilateral ECT the:
-
a seizure threshold is lower
-
b length of the cerebral seizure is greater
-
c length of the generalised convulsion is greater
-
d cerebral seizure is initiated in the left temporal lobe
-
e cerebral seizure is more symmetrical in cortical and subcortical regions.
-
-
5 Which one of these measures in the ECT clinic is the best guide to the adequacy of the induced seizure:
-
a the length of the generalised tonic–clonic convulsion
-
b the length of the cerebral seizure measured by two-channel EEG
-
c the empirically measured seizure threshold
-
d the absolute electrical dose measured in millicoulombs
-
e the extent to which the electrical dose exceeds the seizure threshold.
-
MCQ answers

| 1 | c | 2 | a | 3 | b | 4 | e | 5 | e |






eLetters
No eLetters have been published for this article.