Considered untreatable before the 1960s, obsessive–compulsive disorder (OCD) remains a common and enduring illness that can affect any age group (Reference Skoog and SkoogSkoog 1999). Pharmacotherapy for the disorder began in the 1960s, which saw the introduction of the tricyclic agent clomipramine. Treatment has since evolved, with selective serotonin reuptake inhibitors (SSRIs) in the 1980s and augmentation with antipsychotic medication in the 1990s (for a review see Reference Fineberg and GaleFineberg 2005a). It is now possible to achieve significant improvement in OCD symptoms in the majority of people who receive optimised drug treatment and good care. Nevertheless, symptoms can be missed and patients mistakenly diagnosed with depression or anxiety. Recognition and accurate diagnosis are therefore the cornerstone of effective treatment. The current standard first-line treatment (National Collaborating Centre for Mental Health 2006) is to offer behavioural psychotherapy in the form of exposure and response prevention or medication in the form of a serotonin reuptake inhibitor (SRI). This practice is supported by evidence from randomised controlled trials (RCTs) and meta-analyses. A significant body of evidence supports the long-term efficacy of SSRIs in treatment and relapse prevention, and provides an evaluation of side-effects. The evidence for pharmacotherapy of OCD was first assessed in Advances in 1999 (Reference FinebergFineberg 1999). Here we revisit the topic and consider how evidence-based treatment has evolved in the intervening years.
Aetiology
The serotonin hypothesis
The hypothesis that serotonin is involved in the pathogenesis of OCD was established when it was observed that obsessive–compulsive symptoms were relieved by clomipramine, a potent blocker of serotonin reuptake. Support for this hypothesis is based on drug-response data, which show that clomipramine is superior to other tricyclic anti-depressants, including desipramine (Reference Insel, Mueller and GillinInsel 1985), imipramine (Reference Volavka, Neziroglu and Yaryura-TobiasVolavka 1985), nortriptyline (Reference Thoren, Asberg and CronholmThoren 1980) and amitriptyline (Reference Ananth, Pecknold and van den SteenAnanth 1981).
The serotonin hypothesis was further investigated in studies that favourably compared SSRIs with the noradrenergic tricyclic desipramine (Reference Goodman, Price and DelgadoGoodman 1990) as well as monoamine oxidase inhibitors (MAOIs) such as phenelzine (Reference Jenike, Baer and MinichielloJenike 1997). There have been no convincing studies demonstrating any advantage in using MAOIs. This may be a consequence of their pro-dopaminergic action, which may exacerbate OCD symptoms.
Evaluating treatment response in OCD
Severity of OCD and response to treatment can be evaluated using the Yale–Brown Obsessive–Compulsive Scale (Y-BOCS) (Reference Goodman, Price and RasmussenGoodman 1989) and the Clinical Global Impression-Severity (CGI-S) and Improvement (CGI-I) scales (Reference GuyGuy 1976). Mean change from baseline in Y-BOCS score on active drug compared with placebo can be used to approximate the magnitude of treatment response. Results can also be expressed in terms of response v. non-response to treatment. A reduction in Y-BOCS of 25% or 35% or a CGI-I rating of ‘improved’ or ‘very much improved’ are widely used and appear to separate active from inactive treatments (Reference Simpson, Huppert and PetkovaSimpson 2006).
There is no universally accepted definition of treatment response or remission in OCD, but it has been described as a period during which symptoms have improved to such an extent that they no longer interfere with the individual’s day-to-day life (Reference Simpson, Huppert and PetkovaSimpson 2006). Criteria ranging from a Y-BOCS score < 16 to a score ≤7 have been used in studies to classify remission (Reference Fineberg, Tonnoire and LemmingFineberg 2007a). However, a score of 16 is generally considered to be too high to represent true remission, and a score of 7 too low to be achieved in sufficient cases to be meaningful. Reference Simpson, Huppert and PetkovaSimpson et al (2006) demonstrated that a Y-BOCS score of 7 could not differentiate between active and control treatments. However, two multicentre escitalopram studies showed that a score of 10 could (Reference Fineberg, Tonnoire and LemmingFineberg 2007a).
The evidence base
The standard treatments for OCD that are supported by controlled trials are listed in Table 1.
Clomipramine
Clomipramine was established as an effective treatment for OCD following a case report and double-blind placebo-controlled trials (reviewed in Reference Fineberg and GaleFineberg 2005a). In a comparison with desipramine (Reference Insel, Mueller and GillinInsel 1985), clomipramine produced a significant response in adults, whereas desipramine was indistinguishable from placebo. Similar results have been found in children (Reference Leonard, Swedo and RapoportLeonard 1989). In another small placebo-controlled trial, of the 16 patients receiving clomipramine, 11 showed improvement and none worsened, compared with 2 improving and two worsening on placebo (Reference Greist, Jefferson and RosenfeldGreist 1990).
TABLE 1 Treatments for obsessive–compulsive disorder supported by placebo-controlled trials
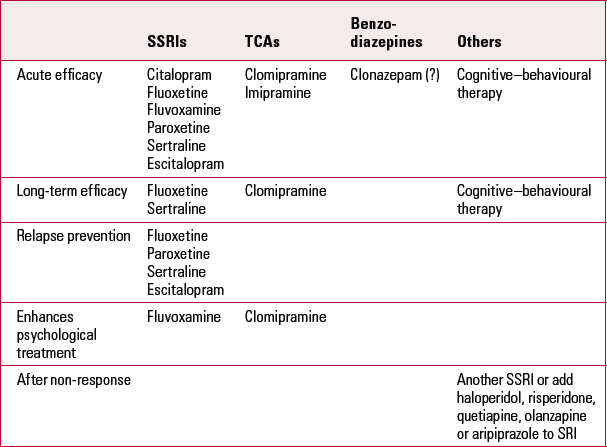
Larger, multicentre studies have assessed clomipramine’s effects in heterogeneous groups of patients with OCD. Two trials which excluded comorbid depression (Clomipramine Collaborative Study Group 1991) demonstrated reduced Y-BOCS scores by 38% and 44% respectively.
Few studies have specifically investigated OCD with comorbid depression, although research shows that at least two-thirds of patients with OCD experience depression at some time. Depression has a significant additional adverse impact on the quality of life of individuals with OCD (Reference Richter, Summerfeldt and AntonyRichter 2003). A study by Reference Thoren, Asberg and CronholmThoren et al (1980) reported significant improvement with clomipramine over nortriptyline and placebo on the obsessive–compulsive subscale of the Comprehensive Psychopathological Rating Scale (CPRS-OC). Reference Insel, Murphy and CohenInsel et al (1983) demonstrated clomipramine’s superiority over clorgyline and placebo. Similar reductions of obsessional symptoms have been reported in other studies in which depression was included (Reference Marks, Stern and MawsonMarks 1980; Reference Mavissakalian, Turner and MichelsonMavissakalian 1985) but the impact of clomipramine on depression was not described.
Fluvoxamine
Results for fluvoxamine have been mixed, with some small studies showing efficacy compared with placebo (Reference Cottraux, Mollard and BouvardCottraux 1990) but others not. Two multicentre trials described improvement in Y-BOCS scores (Reference Goodman, Kozak and LiebowitzGoodman 1996; Reference Hollander, Koran and GoodmanHollander 2003a). Reference Hollander, Koran and GoodmanHollander et al (2003a) used controlled-release fluvoxamine, and found a 32% improvement in Y-BOCS score compared with 21% in the placebo group. Remission (defined as Y-BOCS score < 16) occurred in 44% of participants, but there was a high drop-out rate in the fluvoxamine group, mainly because of side-effects. Superiority of fluvoxamine over desipramine has been demonstrated for both Y-BOCS and Hamilton Rating Scale for Depression scores in patients with OCD and depression, illustrating the significance of treating comorbid depression with an SSRI.
Fluvoxamine plus CBT
Reference Rufer, Hand and AlslebenRufer et al (2005) demonstrated that initial response to cognitive–behavioural therapy (CBT) plus fluvoxamine in 30 people originally treated in hospital for severe OCD was sustained at follow-up 6–8 years after treatment. Response rates (defined as 35% reduction in Y-BOCS score) of 60% were reported and by the end of the study 27% of patients no longer met criteria for OCD (Y-BOCS score < 7) and had entered remission. However, 29 of the 30 had required further medication and/or CBT at some time during the follow-up.
Fluoxetine
Reference Montgomery, McIntyre and OsterheiderMontgomery et al (1993) compared three fixed daily doses of fluoxetine (20 mg, n = 52; 40 mg, n = 52; 60 mg, n = 54). The 20 mg dose did not separate from placebo, but the 40 mg dose outperformed placebo (>25% reduction in Y-BOCS score and CGI-I ‘improved’ or ‘very much improved’). The 60 mg dose showed a significant additional reduction in Y-BOCS score compared with the other doses and placebo. There was no significant difference in the reported side-effects between the groups. In a larger, fixed-dose double-blind RCT (n = 355), all doses were significantly better than placebo, with a trend towards superiority for the high (60 mg) dose (Reference Tollefson, Rampey and PotvinTollefson 1994). Reference Jenike, Baer and MinichielloJenike et al (1997) showed efficacy for fluoxetine but not phenelzine in a comparison of fluoxetine (80 mg/day, n = 23), phenelzine (60 mg/day, n = 20) and placebo (n=21).
Sertraline
Studies using accepted measures such as the Y-BOCS, CGI and National Institute of Mental Health Global Obsessive–Compulsive Scale (NIMH Global OC) have demonstrated efficacy for sertraline (Reference Kronig, Apter and AsnisKronig 1999). One large study (Reference Greist, Jefferson and KobakGreist 1995a) had high drop-out rates in some of the fixed-dose treatment arms over the 1-year treatment period. Pooled data showed that sertraline only just outperformed placebo, with a 39% improvement in Y-BOCS scale compared with 30% for placebo.
Paroxetine
A comparison of paroxetine, clomipramine and placebo found paroxetine to be significantly more effective than placebo and of comparable efficacy to clomipramine (Reference Zohar and JudgeZohar 1996). In another study (Reference Hollander, Allen and SteinerHollander 2003b), higher doses of paroxetine (40 and 60 mg) significantly outperformed placebo, with the 20 mg dose showing no difference. These results suggest that the minimum effective dose of paroxetine is 40 mg/day. Efficacy of a 40 mg fixed-dose against placebo has been shown up to 24 weeks (Reference Stein, Andersen and TonnoirStein 2007).
Citalopram
In a multinational study, fixed-dose citalopram showed an advantage over placebo, with the highest dose (60 mg/day) having the earliest effect (by week 3) (Reference Montgomery, Kasper and SteinMontgomery 2001). The drug was well tolerated, with only 4% of withdrawals being due to adverse events. As well as significant improvement on the Y-BOCS, improvement in psychosocial function was demonstrated on the Sheehan Disability Scale.
Escitalopram
Escitalopram is an SSRI with a dual action on the serotonin transporter: it is thought to enhance its own binding via an additional interaction with an allosteric site on the transporter (Reference Sánchez, Bøgesø and EbertSánchez 2004). In a multicentre, active-referenced (paroxetine) study patients received escitalopram (10 mg/day, n = 112; 20 mg/day, n = 114), paroxetine (40 mg/day, n = 116) or placebo (n = 113) (Reference Stein, Andersen and TonnoirStein 2007). At 12 weeks, higher-dose escitalopram and paroxetine were superior to placebo, and at 24 weeks all three active treatments were superior. The 20 mg escitalopram dose was more effective than the 10 mg dose, compared with placebo. This study demonstrated the importance of continuing treatment for a sufficient period of time and showed that escitalopram (10–20 mg) is an effective treatment, with the 20 mg dose having an earlier onset of action than the 10 mg dose.
Children and adolescents
Post-streptococcal autoimmunity
It has been hypothesised that infection-triggered autoimmune processes are associated with some cases of childhood-onset OCD. The term ‘paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections’ (PANDAS) describes these cases, which resemble Sydenham’s chorea (chorea minor) and demonstrate an acute onset after group A beta-haemolytic streptococcal infection, with neurological signs and an episodic course. PANDAS remains a controversial diagnostic concept, but it has stimulated new research into possible links between bacterial pathogens, autoimmune reactions and neuropsychiatric symptoms. The proposed mediators are anti-basal ganglia antibodies (ABGA). Magnetic resonance imaging supports the proposed pathology, with evidence of inflammatory changes in basal ganglia in cases of PANDAS.
An examination of children with idiopathic OCD revealed that mean ABGA binding was elevated in the patient cohort (n = 50) compared with paediatric autoimmune (n=50), neurological (n=100) and streptococcal (n=40) control groups (P < 0.005 in all comparisons) (Reference Dale, Heyman and GiovannoniDale 2005). This supports the hypothesis that autoimmunity affecting the central nervous system may have a role in a significant subgroup of cases of OCD.
Clomipramine
In many cases, OCD first appears in childhood or early adolescence. Reference Flament, Rapoport and BergFlament et al (1985) were the first to demonstrate the efficacy of clomipramine for childhood OCD. In a subsequent multicentre study involving children and adolescents with OCD, a 37% improvement in Children’s Yale–Brown Obsessive–Compulsive Scale (CY-BOCS) score was recorded with clomipramine, compared with an 8% improvement with placebo (Reference De Veaugh-Geiss, Moroz and BiedermanDe Veaugh-Geiss 1992). A 10-week double-blind crossover trial showed clomipramine to be superior to desipramine at reducing OCD symptoms (Reference Leonard, Swedo and RapoportLeonard 1989). Response to treatment with clomipramine was not predicted by age at onset, duration and severity of illness, type of symptom, or plasma drug concentrations. In this study, 64% of the participants who received clomipramine as the first active treatment showed signs of relapse during desipramine treatment.
Fluvoxamine
A placebo-controlled multicentre study reported fluvoxamine’s efficacy in children aged 8–17 years, as measured by the CY-BOCS (Reference Riddle, Reeve and Yaryura-TobiasRiddle 2001). It noted significant improvement as early as week 1, which continued to the trial end-point at week 10. Only three participants in the fluvoxamine group failed to complete the trial because of side-effects, suggesting that fluvoxamine has satisfactory efficacy and tolerability in childhood OCD.
Fluoxetine
Fluoxetine also shows superiority over placebo for childhood OCD (Reference Liebowitz, Turner and PiacentiniLiebowitz 2002). A crossover study (Reference Riddle, Scahill and KingRiddle 1992) used fixed doses of 20 mg, but proposed that clinical treatment be started at a lower dose because behavioural activation occurred as an adverse effect in a few children, prompting one to leave the study early because of suicidal ideation. The maximum dose was extended to 80 mg with no withdrawals because of adverse effects, suggesting that fluoxetine is effective across the full dose range in children with OCD.
Sertraline
A large RCT has examined sertraline alone, CBT alone, sertraline combined with CBT, or pill placebo in children and adolescents (Pediatric OCD Treatment Study (POTS) Team 2004). The active treatments were well tolerated but the lack of a matched control treatment for CBT limited conclusions about relative efficacy. Sertraline alone and in combination with CBT was efficacious compared with pill placebo.
An analysis of the pooled data from the childhood OCD studies compared ‘numbers needed to treat’ with ‘numbers needed to harm’Footnote † and revealed a positive risk ratio and no suicidal acts for sertraline in children and adolescents (Reference March, Klee and KremerMarch 2006).
Paroxetine
Paroxetine was well tolerated in a multicentre RCT by Reference Geller, Wagner and EmslieGeller et al (2004), who reported efficacy for the drug (10–50 mg) in children and adolescents 7 years of age and older. However, response rates decreased with increasing psychiatric comorbidity.
Pregnancy
Prospective mothers must be advised of the risks and benefits associated with prescribing in pregnancy, as safety can never be assured. Possible risks include malformation of the fetus (in the first trimester), toxicity (in the third trimester) and withdrawal symptoms in the newborn. This must be balanced against the risk of relapse, which may have a negative effect on the mother and the child if medication is discontinued.
Prescribing guidelines for OCD match general principles for prescribing in pregnancy (National Collaborating Centre for Mental Health 2007):
-
• confirm pregnancy as quickly as possible
-
• choose drugs with lower risk profiles for the mother and fetus
-
• start at the lowest effective dose and slowly increase it
-
• use monotherapy in preference to combinations
-
• monitor the fetus throughout pregnancy
-
• communicate treatment plans and decisions to the obstetric team and other involved healthcare professionals
-
• monitor the neonate for discontinuation effects such as irritability, agitation and seizures
-
• monitor infants of mothers who are taking psychotropic medication while breast-feeding for adverse drug reactions.
Breast-feeding
Much of the available data on the immediate and long-term effects of maternal psychotropic medication on breast-fed infants is in the form of case reports or small studies. General principles include:
-
• prescribe the lowest effective dose
-
• monitor the child for normal development, feeding patterns, behaviour and specific adverse effects of the medication.
Elderly people
Obsessive–compulsive disorder continues into old age. Compulsive symptoms may also arise for the first time later in life as a consequence of neurological insult. The consequences of advancing age include a change in the pharmacokinetics and pharmacodynamics of most drugs; an increase in frequency of adverse events because of changes in drug metabolism and drug–drug interactions; and an increase in polypharmacy owing to the higher incidence of comorbid physical and psychiatric conditions.
Guidelines for treating OCD in older people include:
-
• prescribe only when necessary
-
• start with a low dose and increase as needed but be mindful of undertreating
-
• use drugs that block α1-adrenergic or acetylcholine (ACh) receptors (such as tricyclic antidepressants) with caution, as they are more likely to have side-effects in elderly people.
First-line treatment: SRIs
A drug’s clinical effectiveness is dependent on the balance between efficacy, safety and tolerability.
Efficacy
Analysis of OCD trials must be viewed with caution because of the variation in populations studied, differing methods and the fact that published results span several decades.
The comparative efficacy of treatments is best determined by randomised, head-to-head, double-blind comparison. As already mentioned, several such studies comparing clomipramine with SSRIs have been published (e.g. Reference Zohar and JudgeZohar 1996). On the whole, they have found no evidence for the superior efficacy of any of the SRIs tested thus far. However, SSRIs generally are better tolerated and are associated with fewer serious side-effects compared with clomipramine (see ‘Tolerability’ below).
Meta-analysis has demonstrated the superiority of SRIs over placebo and, in some cases, the superiority of clomipramine over the SSRIs; the SSRIs are essentially each of similar efficacy. However, bias in favour of clomipramine potentially exists as a consequence of variation between studies in factors such as publication year, OCD severity, history of previous unsuccessful treatment, clomipramine-related adverse effects leading to unmasking, and rising placebo-response rates that are observed in later studies. These factors may have increased the power of the earlier studies, which mainly investigated clomipramine, relative to the more recent studies, which mainly investigated SSRIs.
The UK’s National Institute for Health and Clinical Excellence systematically accessed both unpublished and published randomised studies (National Collaborating Centre for Mental Health 2006). Clomipramine and SSRIs were indistinguishable in terms of efficacy, although clomipramine was associated with higher rates of premature trial discontinuation because of adverse events.
A meta-analysis by Geller et al on the pharmaco-therapy of childhood OCD demonstrated results which were consistent with the adult literature (Reference Geller, Biederman and StewartGeller 2003a). The authors recommended that, because of its side-effect profile, clomipramine not be prescribed as a first-line drug for children. A comparison of SRI treatment trials in child and adult OCD found it impossible to discriminate between the efficacy and tolerability of SRIs in the two groups, implying a similar treatment response in both (Reference Fineberg, Heyman and JenkinsFineberg 2004).
Safety
Pharmacokinetic variation must be considered when choosing an SSRI. The P450 enzyme CYP2D6 is inhibited by fluoxetine, paroxetine and, to a lesser extent, sertraline, which affects the metabolism of tricyclic antidepressants, anti-psychotics, antiarrythmics and beta-blockers; CYP1A2 and CYP3A4 are inhibited by fluvoxamine, influencing the elimination of warfarin, tricyclics, benzodiazepines and some antiarrhythmics. For elderly people and those on other medication, citalopram and escitalopram may be preferable, as these are relatively free from hepatic interactions. Fluoxetine, with its long half-life and fewer discontinuation effects, can be beneficial in patients who intermittently forget to take their tablets and in pregnancy, when it is considered to be generally safe, having been extensively used (Reference Emslie and JudgeEmslie 2000).
Tolerability
The side-effects of clomipramine are typical of anticholinergic blockade (dry mouth and constipation) and antihistaminic (H1) binding (sedation and weight gain) and can be predicted from its receptor-binding profile. Other side-effects include orthostatic hypotension, thought to be caused by α-adrenergic blockade, nausea, tremor, impotence, anorgasmia and, in up to 80% of patients, impairment of sexual performance. Compared with the SSRIs, clomipramine is associated with a greater risk of potentially dangerous side-effects such as prolongation of the QT interval and seizures. The potential lethality of clomipramine when taken in overdose needs to be borne in mind when prescribing for OCD, in view of the elevated suicide risk associated with the illness. Comorbid OCD in people with bipolar disorder has been cited at rates as high as 30%. Although SSRIs are considered less likely than clomipramine to precipitate mania, mood-stabilising medication is still advised in this group of patients (Reference Kaplan and HollanderKaplan 2003).
The SSRIs are generally safe and well tolerated: placebo-controlled trials report adverse-event-related withdrawal rates between 5% and 15%. Transient side-effects include increased nausea, nervousness, insomnia, somnolence, dizziness and diarrhoea. Sexual side-effects such as reduced libido and delayed orgasm affect up to 30% of participants. The effectiveness of SSRIs has been compared and no superior efficacy of any individual compound found (Reference Bergeron, Ravindran and ChaputBergeron 2002).
Dosage
Dose–response relationship
Reference MontgomeryMontgomery (1980) showed efficacy for a relatively low fixed dose of 75 mg clomipramine. Studies suggest that fluoxetine and paroxetine are more efficacious at higher doses: at 20 mg, paroxetine could not be differentiated from placebo (Reference Hollander, Allen and SteinerHollander 2003b; Reference Stein, Andersen and TonnoirStein 2007). Evidence for superiority of 60 mg doses of citalopram appeared only following secondary analyses (Reference Montgomery, Kasper and SteinMontgomery 2001). Reference Greist, Chouinard and DuBoffGreist et al (1995b) failed to find a dose–response relationship for sertraline in a dose-finding study, although the trial may have been underpowered. Reference Stein, Andersen and TonnoirStein et al (2007) demonstrated clear superiority of a 20 mg dose of escitalopram over placebo at 6 weeks that continued to 24 weeks; the 10 mg dose separated from placebo at 16 weeks. These results provide a persuasive argument for a dose–response relationship in OCD.
Dose titration
The benefits of rapid upwards titration of SRIs have not been established, despite evidence that it may produce an earlier response. In a double-blind study, intravenous (but not oral) administration of clomipramine initially produced a large and rapid decrease in obsessive symptoms, possibly related to greater bioavailability, although this advantage was not maintained (Reference Koran, Sallee and PallantiKoran 1997). Comparison of rapid (over 5 days) with slower (over 15 days) escalation of oral sertraline to 150 mg found an initial advantage of rapid titration that disappeared after the sixth week (Reference Bogetto, Albert and MainaBogetto 2002).
Adverse effects of SRIs can be minimised by slow dose increases over weeks and months. The arguments for slower titration are particularly convincing in children and elderly people. Patients with comorbid conditions such as panic disorder must be treated with care, as they may be especially sensitive to the anxiogenic effects of SSRIs. Additional doses of a mood stabiliser may be required in patients with bipolar disorder to reduce the risk of switching into mania. Longer-term side-effects are a common cause of poor adherence and medication discontinuation. Expert consensus favours starting with moderate doses and titrating up to maximum if symptoms persist (National Collaborating Centre for Mental Health 2006).
Combinations of SRIs
Some studies have shown combinations of SRIs to be beneficial. For example, in a comparison of citalopram with and without open-label mirtazapine, a significantly earlier treatment response was achieved in those receiving the two drugs (Reference Pallanti, Quercioli and BruscoliPallanti 2004). However, by 8 and 12 weeks there was no difference in efficacy between the groups.
Clomipramine plasma level and electrocardiogram (ECG) monitoring are recommended when combining clomipramine with SSRIs that potentially cross-react at the level of the hepatic microsomes. Citalopram, escitalopram (and to a lesser extent sertraline) are preferred, as they are unlikely to interfere with clomipramine clearance.
First-line alternatives to SRIs
There are few rational first-line alternatives to SRIs in the treatment of OCD. At low doses, venlafaxine, with its predominantly serotonergic action, was not significantly better than placebo (Reference Yaryura-Tobias and NezirogluYaryura-Tobias 1975). At 300 mg/day, venlafaxine showed no significant difference from paroxetine at 60 mg/day, with a response rate of 40% in both groups (Reference Denys, van der Wee and van MegenDenys 2003). Further study of the non-responders found a more favourable response among those switched from venlafaxine to paroxetine than vice versa, generating less certainty for the efficacy of venlafaxine in OCD.
Treatment maintenance
When treatment begins, patients should be advised that the anti-obsessional effect may take several weeks to develop fully. Progress may seem slow, and patients can find it difficult to acknowledge they are making any at all. Having family or friends substantiate improvements is helpful. Side-effects may emerge early, before improvements appear, but tend to abate with time. It is important to allow treatment effects to develop and not discontinue the drug too early, as gains continue to accrue for at least 6 months and in some cases up to 2 years. A trial at the maximum tolerated dose for at least 12 weeks and careful assessment are advisable before judging a drug’s effectiveness.
Response to SRI treatment is characteristically partial in the early stages. However, between 30% and 70% of patients attain a clinically relevant level of improvement. It must be recognised that patients studied in RCTs often come from specialist OCD centres, potentially resulting in lower response rates because of the higher numbers of treatment-resistant patients seen at such sites. Higher rates of response with SRIs are more often seen in open-label studies (which more closely reflect usual clinical practice) than in double-blind placebo-controlled trials, in which patients know that they might not be receiving active treatment. Treatment with open-label escitalopram achieved clinical response in as many as 78% of participants by the 16-week end-point (Reference Fineberg, Pampaloni and PallantiFineberg 2007b).
A more precise marker of treatment success is remission rate, which shows greater sensitivity to change over time. In the multicentre study mentioned earlier (paroxetine v. escitalopram v. placebo; Reference Stein, Andersen and TonnoirStein 2007), participants went into remission throughout the treatment period, so that by the end-point at 24 weeks about 40% had Y-BOCS scores < 10. Using the same criterion, around 45% of participants achieved remission during a 16-week open-label study of escitalopram at doses of 10 and 20 mg (Reference Fineberg, Pampaloni and PallantiFineberg 2007b). Therefore, the first-line treatment led to meaningful improvement or remission in about 75% and 45% of cases respectively in these clinical trial populations.
Medication that is effective over the long term is desirable when treating chronic OCD. The benefits of continuing with medication in those who respond to acute treatment have been demonstrated in double-blind studies lasting up to 12 months. Patients treated with fluoxetine (20, 40 or 60 mg) continued to improve, although additional and significant improvements were evident only in the 60 mg group, which suggests that remaining at the higher dose confers additional benefit (Reference Romano, Goodman and TamuraRomano 2001). In another study (Reference Greist, Jefferson and KobakGreist 1995a), patients who responded to initial treatment with sertraline or placebo continued under double-blind conditions for a further 40 weeks. Those remaining on sertraline showed sustained improvements and reduced side-effects with time. Some were followed up for a second year, taking sertraline in an open-label manner. They showed additional improvements in symptoms and a lower incidence of side-effects compared with the earlier study. These results suggest that longer-term treatment continues to be effective, with benefits accruing over time. Dose reduction is not supported.
Relapse prevention
In studies, patients who were switched from clomipramine to placebo showed a rapid and incremental worsening of symptoms (Reference Flament, Rapoport and BergFlament 1985). This implies that, to remain effective, treatment should be continued. Relapse-prevention studies have shown mixed results, mainly because of differing methods and definitions of relapse (Reference Fineberg, Pampaloni and PallantiFineberg 2007b).
A study of adult responders to 12 weeks of open-label paroxetine (20–60 mg) demonstrated a significant advantage for those remaining on the active drug (38% relapse v. 59% on placebo) over the 6-month randomisation phase (Reference Hollander, Allen and SteinerHollander 2003b). In a study of children and adolescents (Reference Geller, Biederman and StewartGeller 2003b), relapse rates in the paroxetine group (34.7%) did not differ significantly from those in the placebo group (49.3%), possibly because of the short duration (16 weeks) of follow-up. In an unpublished (and arguably underpowered) study by Bailer et al (peer-reviewed for methodological adequacy by the NICE Guideline Group; National Collaborating Centre for Mental Health 2006), paroxetine did not separate from placebo on the selected relapse criterion, although there was a significant advantage for paroxetine on Y-BOCS scores.
Patients continuing on high doses of fluoxetine (60 mg), but not lower fixed doses, showed significantly lower relapse rates than those on placebo, which implies an ongoing advantage for remaining at a higher dosage (Reference Romano, Goodman and TamuraRomano 2001). However, the study did not discriminate between continuation of pooled fluoxetine and discontinuation. A study by Reference Koran, Hackett and RubinKoran et al (2002) did not demonstrate a significant advantage for sertraline in preventing relapse, probably because the criterion for relapse was too strictly defined. In contrast, in an escitalopram relapse-prevention study, patients randomised to placebo relapsed significantly earlier and in greater numbers (52% v. 23% on escitalopram; Reference Fineberg, Tonnoire and LemmingFineberg 2007a). A meta-analysis that included the adulthood OCD studies (Reference Fineberg, Pampaloni and PallantiFineberg 2007b) found a significant advantage associated with remaining on SSRIs as opposed to discontinuation.
These results suggest that relapse prevention is a realistic target in the treatment of OCD. They support the premise that continuing an SSRI can protect patients from relapse. This emphasises the importance of maintaining treatment at an effective dose over the long term. Unfortunately, around a quarter of people with OCD become unwell again, regardless of adherence to treatment, leaving scope to develop better strategies. Treatment after relapse is associated with the possibility of a poorer response, which must be borne in mind when discontinuing medication or continuing at a lower dose.
Discontinuation
Abrupt discontinuation of clomipramine and SSRIs is associated with adverse events. Symptoms most commonly reported are flu-like symptoms, vertigo/dizziness, insomnia, vivid dreams, irritability and headaches. These can last from several days to 3 weeks. However, no clinically significant events have been recorded. Discontinuation of fluoxetine results in relatively fewer reports of adverse effects, probably reflecting the long half-life of the parent drug and its metabolite. Gradual tapering is recommended for all SRIs other than fluoxetine, to reduce the risk of a discontinuation syndrome.
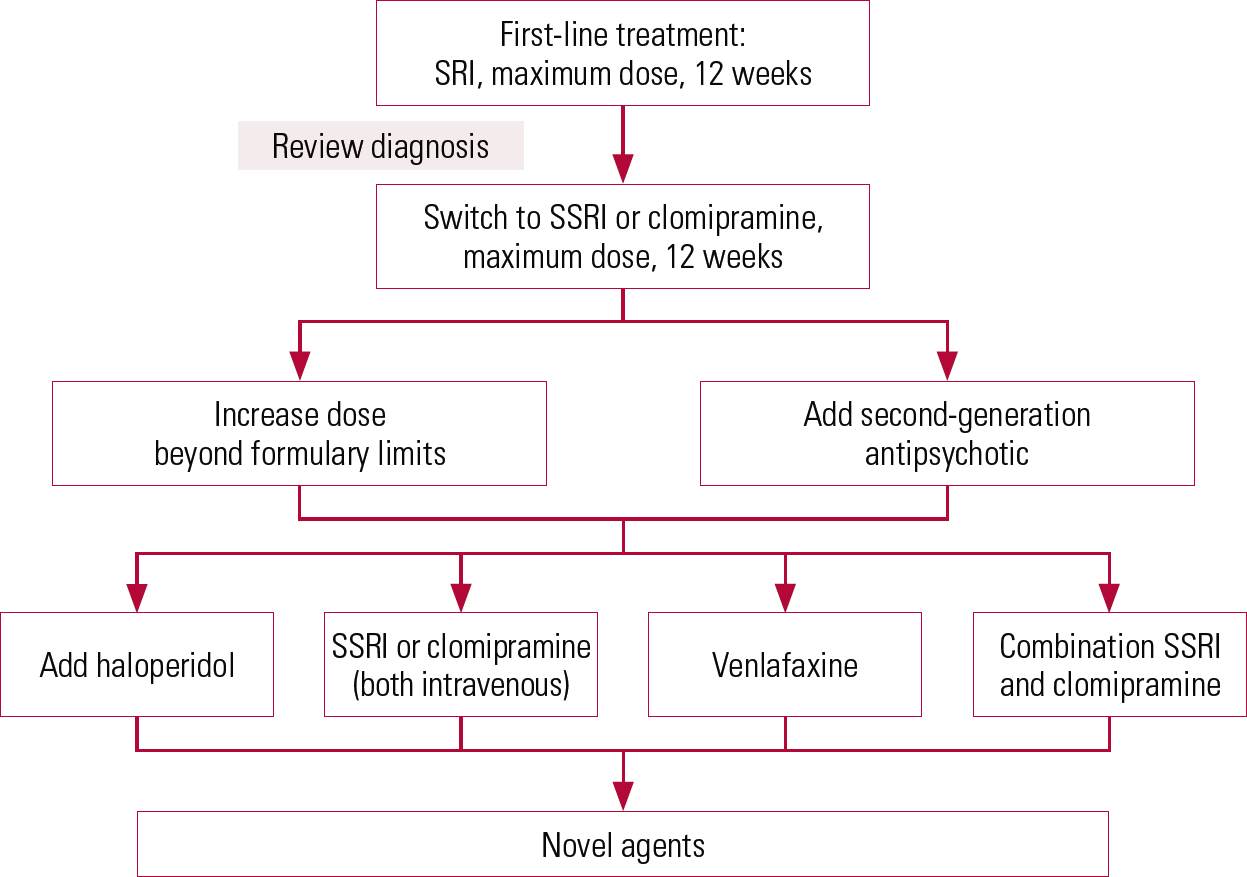
FIG 1 Suggested pathway for treatment-resistant obsessive–compulsive disorder. SRI, serotonin reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor
Treatment resistance
Research into treatment-resistant OCD has been hampered by the lack of consensus on a definition. Confounding can be introduced into treatment trials, particularly those in which a second treatment is added to the first, as patients may respond partially but slowly to first-line treatments. If resistance criteria are applied too strictly, patients may be deemed to be so refractory that they respond to no treatment at all. Therefore, critical to any study investigating strategies for augmentation is a clear and universally accepted definition of treatment resistance. One proposition for a clinically meaningful definition is failure to improve by 25% from baseline Y-BOCS score following treatment with at least two SRIs given at the highest tolerated dose – within the manufacturer’s summary of product characteristics (SPC) recommendations – for a minimum of 12 weeks (Reference Fineberg, Tonnoire and LemmingFineberg 2006a).
Non-response to an adequate trial of an SRI can be accounted for by different factors. An estimate of adherence to treatment (using pill counts or plasma levels) is helpful, although people with OCD are generally adherent unless obsessive–compulsive symptoms interfere. Compared with other symptom dimensions, compulsive hoardingFootnote ‡ has been shown to respond poorly in SSRI trials (Reference Stein, Carey and LochnerStein 2008). Other factors, such as early onset, longer duration, more severe illness and poor response to previous therapy, are linked to poor response to SRIs in adults. In childhood OCD, comorbidity with a number of other psychiatric conditions, such as depression, tics, conduct disorder and attention-deficit hyperactivity disorder, has been associated with treatment resistance.
The remainder of this section discusses options for treatment-resistant OCD (Fig. 1).
Supra-formulary dosing
Selective serotonin reuptake inhibitors have demonstrated a positive dose–response relationship and are well tolerated up to maximum SPC dose limits. It is surprising, therefore, that few studies have examined treatment at higher doses for resistant OCD. Open-label case studies have been reported for citalopram (160 mg/day; Reference Bejerot and BodlundBejerot 1998) and sertraline (400 mg/day); Reference Byerly, Goodman and ChristensenByerly 1996). A placebo-controlled study in patients with resistant OCD not responsive to normal doses showed a better response on the Y-BOCS and CGI-I with high-dose sertraline (250–400 mg/day; Reference Ninan, Koran and KievNinan 2006). However, there was no significant difference in responder rates between the 200 mg/day and the high-dose sertraline groups. Risks with higher doses of clomipramine are greater because of its inherent toxicity. It has been found to be safe at doses up to 300 mg/day (Clomipramine Collaborative Study Group 1991). In the UK it is licensed up to 250 mg/day. Other strategies are usually preferable to exceeding the licensed maximum dose of clomipramine, but if clomipramine is administered above the licensed maximum, ECG and plasma monitoring are advisable, given the drug’s potential for cardiotoxicity and seizures (in 2% of patients).
Intravenous treatment
A strategy to overcome treatment resistance is to alter the mode of drug delivery. A recent review (Reference Ravindran, Jung and RavindranRavindran 2010) reported double-blind trials lending support to the efficacy of intravenous clomipramine. This technique is available in only a few research settings and limited evidence of long-term benefit, together with practical difficulties in administering a compound intravenously in a general psychiatric setting, are the main disadvantages. Similar findings were reported in an open-label trial of intravenous citalopram, the results of which require substantiation under double-blind conditions.
Switching
A literature review showed that some patients not responding to the first SRI showed clinically meaningful response to a second one (Reference Fineberg, Nigam and SivakumaranFineberg 2006b). However, it has also been suggested that switching medication should be delayed until an adequate trial (8–12 weeks at the maximum tolerated dose) has been attempted (Reference March, Frances and KahnMarch 1997). Other evidence supports extending treatment with the initial drug in preference to changing to other treatment strategies. Reference Diniz, Fossaluza and de Braganga PereiraDiniz et al (2011) randomised patients who had not responded adequately to 8 weeks of fluoxetine to receive either clomipramine or quetiapine augmentation or placebo in addition to continuing fluoxetine for a further 12 weeks. Those who received adjunctive clomipramine or placebo responded significantly better than those who received adjunctive quetiapine, supporting the practice of prolonging treatment with an SSRI before augmenting with another agent. A small amount of open-label study data suggest that there is benefit in changing to venlafaxine in patients not responding to one or more SRIs. However, a study by Reference Denys, van Megen and van der WeeDenys et al (2004a) casts doubt on this, as it shows less benefit from switching SSRI non-responders to venlafaxine than venlafaxine non-responders to an SSRI.
Augmentation
If patients fail to show sufficient improvement after two consecutive trials with different SRIs, the addition to the SRI of a drug from a different class should be considered.
SRI plus antipsychotic
Dopamine antagonists release neurons from dopaminergic inhibition and may as a consequence have an indirect serotonergic effect. By today’s standards, there are no positive clinical trials of antipsychotics as monotherapy. An open-label trial of clozapine given as a monotherapy to 12 adults with resistant OCD showed no effect (Reference McDougle, Barr and GoodmanMcDougle 1995). Conversely, aripiprazole (10–30 mg) given as monotherapy in an open-label study involving drug-naive as well as drug-resistant patients showed some hint of a possible clinical benefit (Reference Connor, Payne and GaddeConnor 2005).
Evidence from an increasing number of RCTs supports augmentation with haloperidol, risperidone, olanzapine or quetiapine. Meta-analysis of adjunctive antipsychotic trials has suggested overall efficacy for this approach (Reference Bloch, Landeros-Weisenberger and KelmendiBloch 2006); an antipsychotic plus an SRI was particularly effective in patients with comorbid tic disorder.
First-generation (typical) antipsychotics
McDougle and colleagues have conducted several fluvoxamine augmentation trials. The addition of open-label pimozide to the treatment of patients not responding to fluvoxamine showed some benefit, particularly in individuals with comorbid chronic tics or schizotypal disorder (Reference McDougle, Goodman and PriceMcDougle 1990). A subsequent double-blind placebo-controlled study (Reference McDougle, Goodman and LeckmanMcDougle 1994) reported significant improvement in Y-BOCS scores with haloperidol (2 mg/day) added to fluvoxamine. Mean Y-BOCS score reduction on haloperidol was 26%, and 64% of patients achieved a strict ‘responder’ status, compared with none on placebo. Again, patients with comorbid tics demonstrated a stronger response. Twenty-nine per cent of participants taking haloperidol experienced extrapyramidal side-effects, emphasising the need for balance between efficacy and side-effects when prescribing this drug.
Second-generation (atypical) antipsychotics
A broader range of neurotransmitters, including serotonin, are affected by second-generation anti-psychotics. The side-effect profile of these drugs is perceived to be more benign than that of the first-generation agents and they are increasingly being used in treatment-resistant OCD.
It is not clear why dopamine antagonists might have anti-obsessional properties when combined with SRIs but not when used as mono-therapy. Indeed, some authors have reported emergent obsessions arising during treatment of schizophrenia with atypical antipsychotics. This may be related to their mixed receptor antagonist properties. However, this conclusion remains to be confirmed in prospectively designed studies.
Risperidone
A 12-week double-blind study involving patients who did not respond to at least 12 weeks’ treatment with an SRI demonstrated that addition of risperidone (2.2 mg/day) to an SSRI was superior to placebo in reducing mean Y-BOCS scores, in addition to improving symptoms of anxiety and depression (Reference McDougle, Epperson and PeltonMcDougle 2000). Fifty per cent of those who completed the study were nominated responders. In another study, 45 drug-naive patients were enrolled in a 12-week open-label fluvoxamine trial (Reference Erzegovesi, Guglielmo and SiliprandiErzegovesi 2005). At 12 weeks, 10 non-responders were randomised to 6 weeks of low-dose (0.5 mg) risperidone augmentation and 10 to placebo augmentation. No between-group analysis was carried out, but five individuals treated with risperidone responded, compared with two on placebo.
Quetiapine
In an RCT, 21 people who had DSM-IV OCD and no significant Axis I comorbidity were given an SRI plus quetiapine (≤400 mg) or an SRI plus placebo after they had failed to respond to at least 6 months of SRI treatment (Reference Fineberg, Sivakumaran and RobertsFineberg 2005b). At 16 weeks, there was no significant difference between groups (change in Y-BOCS score from baseline), although 27% of those treated with quetiapine were classed as responders.
Another quetiapine augmentation study reported significant improvement in both the treatment and the placebo groups but no significant difference between the two (Reference Carey, Vythilingum and SeedatCarey 2005). However, the authors identified a number of limitations to the study that might have artificially inflated the placebo rate and reduced the study’s power. These include an inadequate duration of treatment with an SRI before entering the study, a less refractory study population and the possibility that too frequent assessment boosted the placebo response rate. Efficacy for adjunctive quetiapine (< 300 mg) was reported in a double-blind, placebo-controlled study in a sample of 40 patients who failed to respond to at least two SRIs (Reference Denys, De Geus and van MegenDenys 2004b); intention-to-treat analysis showed a significant advantage for quetiapine over placebo from as early as 4 weeks.
A meta-analysis of the three quetiapine studies found evidence supporting efficacy of adjunctive quetiapine at < 400 mg/day (Reference Fineberg, Stein and PremkumarFineberg 2006c). The analysis was limited by the heterogeneity of the studies. Pooled analysis of the same data also showed superior response in the quetiapine-treated group compared with the placebo group (Reference Denys, Fineberg and CareyDenys 2007).
Olanzapine
Twenty-six people with OCD unresponsive to at least two 12-week trials of SRIs and one trial of behavioural therapy were given olanzapine in a double-blind, placebo-controlled study (Reference Bystritsky, Ackerman and RosenBystritsky 2004). The drug was well-tolerated and significantly superior to placebo. No additional advantage was demonstrated for augmentation with olanzapine when fluoxetine plus olanzapine was compared with fluoxetine plus placebo in patients showing no or partial response to pre-treatment with open-label fluoxetine (Reference Shapira, Ward and MandokiShapira 2004).
Haloperidol and sulpiride
First-line treatments for Tourette’s syndrome include the antipsychotics haloperidol and sulpiride, and efficacy of these in OCD supports a theoretical link between the two disorders (Reference McDougle, Walsh, Fineberg, Marazitti and SteinMcDougle 2001). Dose-ranging and long-term studies are required to test relative efficacy, sustained efficacy, tolerability and relapse prevention. Treatment is usually started at low doses in light of potential adverse effects and increased subject to tolerability. A high level of relapse following discontinuation has been reported in a small open-label study (reviewed in Reference McDougle, Walsh, Fineberg, Marazitti and SteinMcDougle 2001).
Adjunctive behavioural therapy
A combination of an SRI and exposure and response prevention is considered superior to either treatment given alone, although few controlled studies have addressed this. Evidence for enhanced efficacy of clomipramine and exposure therapy is inconsistent. Fluvoxamine has been shown to enhance the efficacy of exposure therapy (Reference Cottraux, Mollard and BouvardCottraux 1990) and multi-modal CBT (Reference Hohagen, Winkelmann and Rasche-RüchleHohagen 1998). Some studies suggest that relapse rates are lower after initial treatment with a psychological rather than a pharmacological intervention. One of the difficulties of assessing CBT in controlled trials is the generation of an effective placebo to the CBT.
Reference Simpson, Foa and LiebowitzSimpson et al (2008) conducted an RCT of an SRI augmented with either exposure and response prevention therapy or stress management training. Patients with Y-BOCS score ≥ 16 despite 12 weeks’ treatment with an SRI received either 17 sessions of exposure and response prevention (n = 54) or stress management (n = 54) twice a week while continuing SRI therapy. Of the 108 entrants, 94 completed the course of CBT, with no significant between-group difference in drop-out rates. By week 8, significantly more patients receiving exposure and response prevention therapy had Y-BOCS scores ≥ 25% lower than baseline, with a mean reduction in Y-BOCS of 44% in the treatment group and 14% in the placebo group. This indicates that augmenting SRI pharmacotherapy with exposure and response prevention CBT is an effective strategy for the reduction of OCD symptoms in individuals who partially respond to SRI monotherapy.
Novel treatments
Inositol
Few of the novel treatments proposed and tested for treatment-resistant OCD have shown promise. Small studies have shown inconsistent results for inositol in OCD and putative OCD-spectrum disorders. Dietary inositol is incorporated into neuronal cell membranes as inositol phospholipids, where it serves as a key metabolic precursor in G protein-coupled receptors. Inositol reverses 5-HT receptor desensitisation and modulates the interaction between neurotransmitters, drugs, signalling proteins and receptors, including the 5-HT receptor (Reference LevineLevine 1997), which might affect the experience of OCD symptoms.
Neuropeptides
Neuropeptides influence many functions relevant to OCD, such as memory, grooming, sexual and aggressive behaviour and stereotyped behaviour. There is also significant interaction between neuropeptides and the monoamine system. An increasing body of literature suggests that neuropeptide abnormalities contribute in OCD (for a review see Reference McDougle, Barr and GoodmanMcDougle 1999). However, there have been few treatment trials, possibly because of difficulties in administering these compounds.
Trials of oxytocin, which has an attenuating effect on memory consolidation and retrieval, yielded little success in OCD. Side-effects included psychosis, memory impairment and hyponatraemia. One possible explanation is that a limited amount of peripherally administered oxytocin crosses the blood–brain barrier (Reference Mens, Laczi and TonnaerMens 1983).
Glutamate moderation
Evidence to support the proposition that abnormal glutamatergic transmission is important in OCD is emerging from several areas of research, from neuroimaging to genetics (for a review see Reference Pittenger, Krystal and CoricPittenger 2006). A neuroimaging study found that children with OCD had abnormally high glutamatergic concentrations that decreased with SSRI treatment, in line with symptom severity (Reference Rosenberg, MacMaster and KeshavanRosenberg 2000). These findings have sparked a number of case reports and open-label series involving drugs that modulate glutamate.
Reports of an immediate beneficial effect of augmentation with the glutamatergic compound memantine led to the exploration of adjunctive memantine in a number of small uncontrolled studies that have shown promising results (Reference Aboujaoude, Barry and GamelAboujaoude 2009; Reference Feusner, Kerwin and SaxenaFeusner 2009). These results suggest that memantine may have preferential efficacy in the treatment of OCD v. generalised anxiety disorder. Reference Stewart, Jenike and HezelStewart (2010) conducted a further single-blind case–control study of memantine for severe OCD. Twenty-two in-patients receiving memantine in addition to standard care were matched with 22 controls receiving standard care alone. The mean reduction in Y-BOCS score was 7.2 (27%) for memantine and 4.6 (16.5%) for standard care, providing further evidence for the possible efficacy of adjunctive memantine in OCD.
Riluzole, another glutamate antagonist, produced positive effects as an adjunctive agent in a small open-label trial in adults (Reference Coric, Taskiran and PittengerCoric 2005). Two further small open-label studies of riluzole in children with OCD reported improvement on the Y-BOCS without major adverse effects (Reference Grant, Lougee and HirschtrittGrant 2007; Reference Pittenger, Coric and BanasrPittenger 2008). These findings have been supported by preliminary results of an ongoing trial (Reference Grant, Song and SwedoGrant 2010), despite the occurrence of two cases of pancreatitis.
d-cycloserine is a partial agonist of the N-methyl-d-aspartate (NMDA) receptor. It has been used as an augmentation agent to enhance exposure therapy outcome in paediatric OCD in a randomised double-blind placebo-controlled augmentation trial (Reference Storch, Murphy and GoodmanStorch 2010). Although not statistically significant, the active treatment arm showed small to moderate treatment effects. There have been mixed results with d-cycloserine augmentation of exposure and response prevention therapy in adults (Reference Kushner, Kim and DonahueKushner 2007; Reference Storch, Merlo and BengtsonStorch 2007; Reference Wilhelm, Buhlmann and TolinWilhelm 2008). Only the study by Reference Wilhelm, Buhlmann and TolinWilhelm (2008) yielded positive results, at the mid- rather than the endpoint of the study. Treatment with glycine, another NMDA receptor modulator, in a randomised double-blind placebo-controlled trial (Reference Greenberg, Benedict and DoerferGreenberg 2009) produced a statistically insignificant mean reduction in Y-BOCS score of 6 points, compared with 1 point in the placebo group. The compound was poorly tolerated, mainly because of its aversive taste or nausea.
Ondansetron
Positive results from an open-label study of the 5-HT3 receptor antagonist ondansetron was followed by an 8-week double-blind, placebo-controlled pilot study (Reference Soltani, Sayyah and FeizySoltani 2010). Patients were randomised to receive fluoxetine (20 mg/day) and either ondansetron (4 mg/day) or placebo. At the end-point, the mean Y-BOCS score in the ondansetron group was significantly lower (5 v. 15; baseline in both groups was 35), suggesting possible efficacy in OCD.
Morphine
The efficacy of augmentation with oral morphine has been demonstrated in a double-blind crossover study of patients with a Y-BOCS score ≥ 20 who had failed two or more adequate SRI trials (Reference Koran, Aboujaoude and BullockKoran 2005). Participants were assigned to random-order, 2-week blocks of once-weekly morphine, lorazepam or placebo. After treatment with morphine, 7 participants (30%) were classed as responders (≥ 25% decrease in Y-BOCS score). The analysis showed significance for morphine v. placebo but not for lorazepam v. placebo. Two randomised trials of naloxone have found no significant effect.
Electroconvulsive therapy and transcranial magnetic stimulation
Despite isolated reports of its success in treatment-resistant cases, electroconvulsive therapy (ECT) has not shown consistent evidence of improving core OCD symptoms. It may be appropriate for patients with comorbid severe treatment-resistant depression, particularly those at risk of suicide.
Repetitive transcranial magnetic stimulation (rTMS)Footnote § has potential as a form of therapy and as an instrument to help determine the neurocircuitry involved in disorders such as OCD. In rTMS, a pulsatile high-intensity electromagnetic field emitted from a coil placed against the scalp induces focal electrical currents in the underlying cerebral cortex, stimulating or disrupting cortical activity. Reference Blom, Figee and VulinkBlom et al (2011) reviewed 10 trials of rTMS involving 110 patients. It appears that open-label studies of rTMS applied to the dorsolateral prefrontal cortex, the orbitofrontal cortex and supplementary motor areas resulted in transient reduction in obsessive–compulsive symptoms. In randomised, double-blind, sham-controlled studies there was no significant difference between active and control treatments. The authors concluded that variation in study design with respect to treatment parameters made it difficult to draw clear conclusions. Neuro-navigational techniques may assist in more accurately targeting rTMS to these areas.
NeurosurgeryFootnote ¶
Modern stereotactic neurosurgical techniques (cingulotomy, capsulotomy) for resistant OCD ablate connections between the frontal lobes and subcortical structures and should not be equated with the relatively crude surgical approaches of the past. Studies suggest that modern neurosurgery can offer clinically meaningful symptom relief and improved function for some patients with ‘untreatable’ chronic, severe OCD. However, follow-up studies of greater rigour are required to determine long-term outcomes, including adverse effects (Reference Matthews and EljamelMatthews 2003; Reference Rück, Andréewitch and FlycktRück 2003). Specifically, it is not possible to judge the true efficacy of surgery, the optimal placement of lesions or the longer-term outcome in the absence of masked comparator-controlled follow-up studies. Reference Jung, Kim and ChangJung et al (2006) investigated the efficacy and adverse effects of stereotactic bilateral anterior cingulotomy in 17 individuals with refractory OCD. Participants were evaluated before surgery and at 12 and 24 months after the procedure. The mean improvement on Y-BOCS score was 48% and criteria for response were met in 8 of the 17 participants (47%). No significant adverse effects were noted after surgery or at 24-month follow-up.
Stereotactic neurosurgery should be viewed as the option of last resort, given the irreversible nature of the procedure. It should be considered only for gravely ill patients and only after, as a minimum, well-documented pharmacotherapy (including clomipramine), exposure and response prevention and combination strategies have been tried.
Deep-brain stimulation
Deep-brain stimulation (DBS) is based on neuro-modulation methods. It has been successfully used in the treatment of Parkinson’s disease and serves as an alternative to traditional neurosurgery. The technique involves implanting electrodes that deliver an electric current directly into the neural structures considered to underpin OCD (Reference Krack, Hariz and BaunezKrack 2010). It is not intended to be ablative and is reversible. Response rates of up to 50% have been achieved in small groups of people with highly resistant OCD in uncontrolled studies stimulating targets that include the anterior limb of the internal capsule, limbic part of the subthalamic nucleus and ventral capsule/ventral striatum (V/S) (Reference de Koning, Figee and van den Munckhofde Koning 2011). Overall, average Y-BOCS score decreases range from 6.8 to 31 points. The frequency of adverse events seems to be limited, but DBS is not without risks. Surgical adverse effects reported included an asymptomatic haemorrhage, seizure and superficial infection. Psychiatric adverse effects included transient hypomanic symptoms, worsened depression and OCD when battery depletion interrupted the procedure. Despite this, the results are highly promising given the treatment-resistant nature of the participants. Controlled studies are under way to substantiate efficacy and determine optimal electrode placement, stimulus parameters and long-term effects (Reference de Koning, Figee and van den Munckhofde Koning 2011).
Summary
It should be recognised that the novel treatments discussed above are options if all else fails. It is beyond the scope of this article to suggest a predefined menu of options in the event of failure of initial and subsequent treatments. Suffice to say that consideration should be given to in-patient treatment and referral to specialist services before novel or surgical options are explored.
New treatment services
A small number of UK specialist centres where clinical expertise is concentrated have been nationally commissioned to provide a service for National Health Service patients with severe, complex and treatment-resistant OCD or body dysmorphic disorder (BDD). Called the National OCD/BDD Service (www.swlstg-tr.nhs.uk/our-services/ocd-bdd-service-bcpu-national), this offers in-patient and out-patient treatment for those with very severe disease who have not responded to standard treatments. Additional information is available via www.specialisedservices.nhs.uk and www.advancedinterventions.org.uk.
Conclusions
In OCD, SRIs have a rapid onset and broad spectrum of actions, including the treatment of comorbid conditions such as depression and anxiety (Box 1). The SSRIs are generally safer and better tolerated than clomipramine and are generally used as first-line treatment. They are cost-effective compared with other treatment options (National Collaborating Centre for Mental Health 2006). Greater benefits are usually achieved with higher doses, and gains continue to accrue gradually over weeks and months. The SSRIs achieve a clinically meaningful response in about 70% of patients and remission after sustained treatment in around 40%. Continuing treatment protects against relapse in the majority of cases.
BOX 1 Key clinical points
-
• SRIs are the cornerstone of pharmacological treatment and lead to substantial clinical improvement in the majority of cases
-
• SSRIs are the preferred first-line treatment; clomipramine is a good alternative for those who cannot tolerate or fail to respond to SSRIs
-
• Gradual dose titration upwards within licensed limits, measuring clinical response and side-effects, is appropriate
-
• Treatment at maximally tolerated dose levels for at least 12 weeks is advisable to properly assess effectiveness
-
• Clinical response should be measured objectively using standardised rating scales (e.g. Y-BOCS)
-
• In the majority of patients, symptoms respond only partially to SRIs, and around one-third of patients do not achieve a clinical response
-
• Maintenance treatment appears to protect against relapse
-
• In treatment-resistant OCD, combination CBT plus pharmacotherapy, increasing dosages or switching between SRIs are practical next steps
-
• In refractory OCD, evidence supports the addition of dopamine antagonists at the lower end of their dosing range, although long-term data are lacking and the response remains unsatisfactory for many patients
-
• Novel pharmacological treatments such as drugs acting on the opiate, serotonin and glutamate systems offer promise and are under investigation
In SRI-resistant OCD, evidence is strongest for the use of adjunctive antipsychotics. These are clinically effective in up to two-thirds of patients, particularly those with comorbid tic disorders. Increasing the dose of SSRI or switching SRIs are rational alternatives.
A nationally commissioned service offering in-patient and out-patient treatment for those with very severe disease who have not responded to standard treatments is now available (www.specialisedservices.nhs.uk).
Future directions
Improving the diagnosis and delivery of health-care for patients with OCD must continue to be a high priority. Further research should target new therapies, novel techniques (such as pharmacological functional magnetic resonance imaging and pharmacogenomics) and standardise methods to measure treatment response with sound evidence guiding treatment choices.
MCQs
Select the single best option for each question stem
-
1 The following are not evidence-based treatments for OCD:
-
a clomipramine
-
b escitalopram
-
c agomelatine
-
d fluvoxamine
-
e fluoxetine.
-
-
2 In the treatment of OCD:
-
a clomipramine is usually better tolerated than SSRIs
-
b SSRIs and clomipramine are rarely associated with sexual dysfunction as an adverse effect
-
c the treatment effect of SSRIs emerges quickly, within days rather than weeks
-
d long-term treatment remains effective for most patients
-
e there is no evidence that long-term treatment protects against relapse.
-
-
3 In measuring treatment response in OCD:
-
a quantitative rating scales such as the Y-BOCS are rarely helpful
-
b improvements of around 25% as measured on a quantitative rating scale may be construed as a therapeutic response
-
c an informant is rarely helpful
-
d the dose and duration of treatment are unimportant factors when determining treatment resistance
-
e quality of life measures are not informative.
-
-
4 In treating SSRI-resistant OCD:
-
a adjunctive antipsychotics can be helpful
-
b raising the dose of SSRI beyond SPC recommendations is never indicated
-
c reviewing the diagnosis is a waste of time
-
d adjunctive lithium is usually helpful
-
e high doses of adjunctive antipsychotics are considered appropriate.
-
-
5 The non-pharmacological treatment that shows least evidence of clinical benefit in OCD is:
-
a deep brain stimulation to the subthalamic nucleus
-
b stereotactic cingulotomy
-
c exposure and response prevention
-
d stereotactic capsulotomy
-
e psychoanalytic psychotherapy.
-
MCQ answers

| 1 | c | 2 | d | 3 | b | 4 | a | 5 | e |







eLetters
No eLetters have been published for this article.