LEARNING OBJECTIVES
-
Learn to identify patients with SESD and explore the key role of a developmental illness history or time line
-
Be able to assess both the physical and psychological state of patients with SESD and to identify the risk factors for chronicity
-
Be able to plan a management programme and be aware of the resources available in diverse treatment settings
In an earlier BJPsych Advances article, Reference RobinsonRobinson (2014) introduced the term severe and enduring eating disorders (SEED) to describe patients with severe eating disorders that required the regular attention of a multidisciplinary team. Here we describe another neglected group of patients who are more usually encountered in primary care and general hospital settings and account for disproportionately high healthcare costs (Reference Anderson, Eplov and AndersenAnderson 2013). These patients have severe and enduring somatoform disorders (SESD) (Box 1) and report multiple, diverse physical symptoms over a prolonged period (for the sake of this review 5 or more years), with no relevant organic disease to account for their symptoms, although they may have coexisting medical disorders (Boxes 2 and 3).Footnote a The key word here is relevant.
BOX 1 Definition of somatoform disorders
Somatoform disorders are defined in ICD-10 as ‘repeated presentation of physical symptoms over a number of years, together with persistent requests for medical investigations, in spite of repeated negative findings and reassurances by doctors that the symptoms have no physical basis’ (World Health Organization 1992). In DSM-5 it is acknowledged that the symptoms may or may not be associated with another medical condition (American Psychiatric Association 2013).
BOX 2 Somatoform disorder and disability
A man of 50 was referred to the psychiatric out-patient clinic from his occupational health doctor with symptoms of panic attacks, abdominal pains and tremors of both upper limbs. He was finding it difficult to work as a council gardener because he was convinced that his line manager was talking about him behind his back. He was distressed by his physical sensations and, because of his tremors, he could not use his tools. Examination of his medical records revealed a long history of recurrent physical symptoms such as fatigue, tremors, facial and abdominal pains occurring at times of life stress, usually involving interpersonal problems at work. He had been seen by a psychiatric service in his 20s, when he was diagnosed with dependent, paranoid and avoidant personality traits, and had received drug treatment and marital therapy. His complaints of multiple recurrent physical symptoms had occurred since adolescence and had led to significant use of medical resources and lifetime sickness absence.
BOX 3 Chronic physical illness coexisting with somatoform disorder
A 45-year-old man with mild rheumatoid arthritis but with normal inflammatory markers reported a variety of diverse somatic complaints unrelated to his joints, including abdominal pain, headaches and fatigue. He was extremely introspective and worried excessively about his health and had consulted his general practitioner over 20 times in the previous year. His score on the PHQ-15 was grossly elevated at 25/30 and he was functionally impaired, becoming unable to work as a library assistant. He had been retired on medical grounds because of fibromyalgia. He satisfied diagnostic criteria for a persistent somatoform disorder (which had not been treated) coexisting with an (albeit mild) chronic medical disorder, rheumatoid arthritis.
These patients are often not accepted into psychiatric care because they do not have ‘severe and enduring mental illness’ (SEMI) (Department of Health 1999). However, it has been repeatedly shown that they have significant disabilities, comparable to those seen in severe mental illnesses such as schizophrenia and chronic depression (Reference Kushwaha, Sinha Deb and ChaddaKushwaha 2014; Reference Rask, Rosendal and Fenger-GronRask 2015), and that they also make extraordinary demands on carers (Reference Krishnan, Sood and ChaddaKrishnan 2013). SESD continue to be neglected by both psychiatric and medical services. The main reasons, in our opinion, are shown in Box 4.
BOX 4 Reasons why SESD are neglected
-
The ‘firewall’ that continues to exist between medical and psychiatric services is not merely geographical – it is often initiated at an early stage in medical education
-
Many physicians are not trained to recognise or identify patients with concurrent physical and psychiatric disorders
-
Clinicians generally (for pragmatic reasons) tend to adopt a cross-sectional rather than a developmental or life-course approach (one reason for this is that patients’ notes in modern out-patient clinics rarely include the complete medical record or illness history)
-
‘Somatic attribution’ of symptoms is often a feature of these patients, who may avoid acknowledging psychosocial problems
-
Primary care doctors do not always respond to psychosocial cues, which leads to the avoidance of psychiatric services
-
Medical journals rarely contain narrative accounts of patients’ illnesses
-
There is confusion in the literature about the classification of functional syndromes
Definitions
The term somatoform disorder has been replaced in DSM-5 by somatic symptom disorder (American Psychiatric Association 2013), which represents a ‘consolidation of previously listed diagnoses’ (Reference Dimsdale, Creed and EscobarDimsdale 2013). We will retain the former generic term in this article because most psychiatrists are familiar with it, many physicians are aware of it and use it, and much of the research cited below in this review has been based on DSM-IV criteria (American Psychiatric Association 1994).
There have been two major changes from DSM-IV worth emphasising. First, DSM-5 recognises that somatoform disorders can be associated with another medical condition, and that excessive reliance on medically unexplained symptoms (MUS) is simplistic and reinforces mind–body dualism (Reference SharpeSharpe 2013). Second, it proposes that central to these disorders are distressing thoughts, feelings and behaviours and that health concerns are often paramount and occasionally preoccupying (Reference Rief, Mewes and MartinRief 2010; Reference Riebel, Egloff and WitthoftRiebel 2013). These key characteristics are more ‘psychological’ and will be described later.
One unfortunate feature of this area of medical practice is that most physicians are uncomfortable with psychiatric nosology, often preferring to use terms such as chronic fatigue syndrome, fibromyalgia, irritable bowel syndrome and chronic pelvic pain. Psychiatrists working in this field need to be aware that (a) there is a considerable degree of overlap in the phenomenology of these so-called functional disorders (Reference Kanaan, Lepine and WesselyKanaan 2007), and (b) there has been a recent acknowledgement by rheumatologists that fibromyalgia is a dimensional or continuum disorder (Reference Wolfe, Brahler and HinzWolfe 2013) that shares common characteristics with somatic symptom disorder (DSM-5). Indeed, 40% of people with fibromyalgia in the general population satisfy DSM-5 criteria for somatic symptom disorder, supporting the view that there is considerable overlap between these disorders.
In this article we will demonstrate that SESD are more widespread than is commonly acknowledged and that patients with SESD require the attention of a number of professionals from a wide variety of disciplines whose efforts need to be coordinated and documented.
Indices of severity
Not all patients with somatoform disorders fall into the SEMI category. In a systematic review of course and prognosis in patients with MUS, 10–30% were shown to deteriorate (Reference olde Hartman, Borghuis and Lucassenolde Hartman 2009). The key problem lies in identifying which patients will develop chronic symptoms and disabilities.
Severity is difficult to quantify but measures of service use, functional impairment and incapacity for work are useful proxy measures, as are high costs (Reference Barsky, Orav and BatesBarsky 2005). In a recent 10-year follow-up study in primary care, Reference Rask, Rosendal and Fenger-GronRask et al (2015) found that of patients with a DSM-IV persistent somatoform disorder, a fifth were in receipt of disability benefits and had an increased risk of sick leave compared with controls with a well-defined physical disease. In line with previous studies, many of the patients had chronic illnesses and high psychiatric comorbidity.
Another index of severity that can be easily measured in the clinic is the symptom burden or load, which can be documented with a brief rating scale, the PHQ-15 (Reference Kroenke, Spitzer and WilliamsKroenke 2002). In a population-based study both somatic symptom burden and high health anxiety had important effects on functional impairment and healthcare use (Reference Lee, Creed and MaLee 2015). This suggests that the presence of both psychological features (criterion B under somatic symptom disorder in DSM-5) and bothersome symptoms describes a group with greater impairments (Reference Tomenson, Essau and JacobiTomenson 2013). Useful indices of illness severity include the following:
-
number of physical symptoms or symptom load – as measured on the PHQ-15 (cut-off scores of 5, 10 and 15 represent low, medium and high somatic symptom load) (Reference Kroenke, Spitzer and WilliamsKroenke 2002); it is important to note that self-rated symptom counts should not be used to identify patients with MUS (Reference Carson, Stone and HansenCarson 2015)
-
use of primary care services (e.g. symptoms lasting at least 3 months and leading to loss of function) (Reference Aamland, Malterud and WernerAamland 2014)
-
use of secondary care services (e.g. the top 5% of out-patient attenders by number of appointments (Reference Reid, Wessely and CrayfordReid 2002)
-
number of investigations/tests with negative outcome over a 24-month period.
Illness duration
One landmark study of illness longevity was conducted over 8 years (Reference FinkFink 1992a), but overall there are few follow-up studies of patients with SESD. In previous longitudinal studies of patients who meet the criteria for somatoform disorders, between 22 and 53% still have the disorder at follow-up (Reference Jackson and KroenkeJackson 2008; Reference Steinbrecher and HillerSteinbrecher 2011a). The same is true for patients with hypochondriasis (Reference olde Hartman, Borghuis and Lucassenolde Hartman 2009).
It is important for clinicians to be aware of the chronic remittent nature of SESD. Indeed, there is increasing evidence that so-called functional somatic syndromes are linked through a polysyndromic phenotype (Reference Warren, Clauew and WesselmanWarren 2014) and that patients with somatoform disorders often have extensive histories of functional syndromes such as fibromyalgia and irritable bowel syndrome.
Disorder persistence
The key issue for the clinician is to know what predicts persistence. Factors predicting severity are germane: there is evidence that the number of somatic symptoms at baseline and high health anxiety influence the course of these conditions (Reference Jackson and PassamontiJackson 2005), as do illness perceptions. Recent findings, however, have challenged the view that physical illness attributions are a key component of somatoform disorders. Reference Frostholm, Ornbol and FinkFrostholm et al (2015) concluded that it was important to distinguish between attribution of symptoms on the one hand (which may vary more in somatoform disorders than previously assumed) and cognitive characteristics such as catastrophising and negative illness perceptions (which have been shown to predict disability) on the other (Reference Rief, Nanke and EmmerichRief 2004, Reference Rief and Broadbent2007). In disorders such as chronic fatigue syndrome, fibromyalgia and chronic whiplash, however, where the patient's views may be shaped by patient organisations and the internet, evidence suggests that illness beliefs and expectations for recovery can have a marked effect on outcome (Reference CarrollCarroll 2011; Reference Petrie and WeinmanPetrie 2012).
These findings are consistent with clinical experience that suggests that a combination of (a) the number of physical symptoms or symptom burden, as well as (b) illness beliefs and perceptions are key predictors of chronicity, as measured by poor outcome and high healthcare costs (Reference Frostholm, Petrie and OrnbolFrostholm 2014).
Coexisting personality disorder
Another key predictor of chronicity is a coexisting personality disorder (Reference Bornstein and GoldBornstein 2008) (Table 1). It has recently been shown that patients with borderline personality disorder are more likely to experience pain and rate their pain as more severe than patients with other personality disorders (Reference Biskin, Frankenberg and FitzmauriceBiskin 2014).
TABLE 1 Personality disorders theoretically linked with SESD
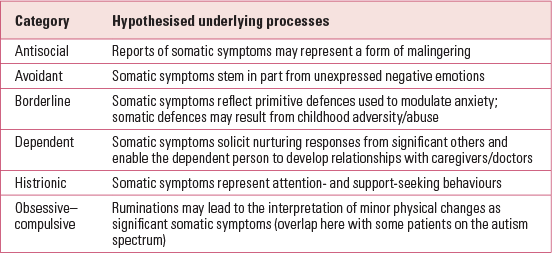
Personality disorder is underdiagnosed in routine clinical practice (Reference Tyrer, Reed and CrawfordTyrer 2015a) and diagnostic accuracy is low, particularly in primary care. To achieve more than an indicative diagnosis, a semi-structured interview is necessary, ideally involving informants and two or more meetings. Patient questionnaires tend to show low agreement with semi-structured interviews (Reference Fossati, Maffei and BagnatoFossati 1998) and are best avoided. The most widely used diagnostic instrument is the Structured Clinical Interview for DSM-IV Axis II Disorders, the SCID-II (Reference Gibbon, Spitzer and FirstGibbon 1997), which takes 1–2 h to administer and is based on the DSM-IV personality disorder criteria, which are unchanged in DSM-5. The equivalent ICD-10 semi-structured patient interview is the International Personality Disorder Examination (IPDE) (Reference Loranger, Jance and SartoriusLoranger 1997), which is slightly longer. Assessments made on a single meeting, and without documentary evidence of former behaviour and difficulties or information from a third party who has known the patient over some time, are likely to be unreliable, and this may explain the reluctance of some clinicians to make the diagnosis (Reference ParisParis 2007).
Personality disorder diagnostic criteria and categories will change substantially in ICD-11, which will introduce a tiered approach to personality dysfunction. It will comprise personality difficulty, which is likely to include the majority of the population, and mild, moderate and severe personality disorders, with qualifiers to indicate the domains within which the difficulties lie (e.g. negative affective, dissocial, anankastic, and detached) (Reference Tyrer, Reed and CrawfordTyrer 2015a).
Symptom substitution
The vignette in Box 5 illustrates that when functional disability reflects unacknowledged psychological distress, results of improvement can have unpredictable psychiatric consequences. Conversely, suicidality and distress can reduce once disability has become established in a patient with SESD (Reference Allanson, Bass and WadeAllanson 2002).
BOX 5 Symptom substitution
A middle-aged woman presented to neurologists with loss of use of her legs. She had a history of functional illnesses. She was admitted for investigation, and when no cause was found a psychiatric consultation was arranged. She responded to brief exploratory treatment and, with physiotherapy, regained the use of her legs. Shortly after discharge she became acutely suicidal and began to self-harm severely. She was admitted to a psychiatric hospital for a prolonged period in an attempt to address her risk. A personality disorder was diagnosed.
Litigation
Litigation is another predictor of chronicity. There is good evidence that those involved in litigation for financial rewards to compensate pain and suffering after traffic injury do not recover as quickly as those that have similar injuries and are not litigating (Reference Cassidy, Bendix and RasmussenCassidy 2011).
Prevalence: how many SESD are out there?
The prevalence of somatic symptom disorders in the general population has been estimated to be 5–7% (Reference Creed, Barsky, Leiknes, Creed, Henningsen and FinkCreed 2011).
In primary care settings the use of different measures and different samples leads to considerable variation in prevalence rates but most studies indicate an overall prevalence of 8–22% (Reference Creed, Barsky, Leiknes, Creed, Henningsen and FinkCreed 2011; Reference Steinbrecher, Koerber and FrieserSteinbrecher 2011b). Persistent somatoform pain disorder is not considered separately from somatoform disorder as the epidemiology is similar (Reference Frohlich, Jacobi and WittchenFrohlich 2006).
Among patients attending a neurology clinic with more severe MUS, persistence over a year occurred in two-thirds and this was associated with the patient's belief that they would not improve, a failure to attribute the symptoms to a psychological cause and the receipt of illness-related financial benefits (Reference Sharpe, Stone and HibberdSharpe 2010).
Clinical features
In a Danish National Patient Register study, Reference FinkFink (1992a) identified patients who, over an 8-year period, had been admitted repeatedly to hospital at least ten times for physical symptoms without an organic basis. He found that 84% were women and three-quarters were under 25 years old at the onset of the disorder. A third had ‘hospital careers’ of more than 20 years and accounted for 3% of the general population's admission to non-psychiatric hospitals. Most patients with somatoform disorders have chronic symptoms and high levels of disability (Reference Bass C MurphyBass 1991), and a high proportion have a coexisting medical disorder (Boxes 2 and 3). Coexisting medical and personality disorders may be associated with complex somatoform disorders, and in these cases assessment can be extremely difficult (Reference Frankenburg and ZanariniFrankenberg 2004). Features that may alert the clinician are shown in Box 6.
BOX 6 Clinical characteristics that may alert the clinician to SESD
-
History of pain-related surgery, especially for similar pains (Reference DeVaul and FaillaceDeVaul 1980)
-
Polysyndromic phenotype, i.e. repeated presentations of functional disorders throughout the life cycle for 5 years or more (Reference Warren, Langenberg and ClauwWarren 2013)
-
Belief/expectations that the symptoms will not improve (Reference CarrollCarroll 2011)
-
Failure to attribute symptoms to a psychosocial cause, i.e. a somatic attributional style despite evidence to the contrary (Reference Frostholm, Petrie and OrnbolFrostholm 2014)
-
In receipt of illness-related financial benefits (Reference Sharpe, Stone and HibberdSharpe 2010)
-
High levels of health anxiety (Reference Lee, Creed and MaLee 2015)
-
High number of somatic symptoms or high symptom burden (Reference Lee, Creed and MaLee 2015)
-
Feeling of lack of control over symptoms (Reference Petrie and WeinmanPetrie 2012)
-
Coexisting personality disorder (Reference Frankenburg and ZanariniFrankenburg 2004; Reference Bornstein and GoldBornstein 2008)
-
Involvement in prolonged litigation related to symptoms (Reference Cassidy, Bendix and RasmussenCassidy 2011)
Psychological features
There is evidence that the onset of SESD is associated with both physical (e.g. muscle trauma) and psychological (e.g. bereavement) triggers that initiate pain amplification and psychological distress (Reference Holliday and McBethHolliday 2011). Psychological features likely to be characteristic of somatoform disorders are shown in Box 7.
BOX 7 Psychological and behavioural characteristics likely to be important in patients with SESD
-
Health anxiety (i.e. ruminating about bodily symptoms)
-
Catastrophic thinking (e.g. these palpitations are causing damage)
-
Self-concept of bodily weakness
-
Intolerance of bodily complaints
-
Avoidance of activity that could raise heart rate or aggravate bodily complaints
-
Disuse of a limb, especially if painful
-
Pain behaviour (e.g. unprescribed wheelchair use)
-
Somatic illness attribution despite contradictory medical information (e.g. attributing multiple diverse symptoms to a physical cause such as lead poisoning, rather than to stressful work situations)
(Reference Rief, Mewes and MartinRief 2010; Reference Voigt, Wollburg and WeinmannVoigt 2013)
Personality disorder
Numerous studies have reported that about three-quarters of patients with somatoform disorders have a coexisting personality disorder. The highest prevalence rates occurred for antisocial, borderline, histrionic and dependent personalities, each of which was diagnosed in around 25% of patients with somatoform disorder (Reference Bornstein and GoldBornstein 2008) (Table 1). Reference Tyrer, Fowler-Dixon and FergusonTyrer et al (1990) identified 2.5% of psychiatric patients sharing stable characteristics of an excessive preoccupation with the maintenance of health, a tendency to overattend to minor physical symptoms and frequent medical consultations. A follow-up study provided support for the poor prognosis in patients with a hypochondriacal personality disorder (Reference Tyrer, Seivewright and SeivewrightTyrer 1999).
Childhood adversity
Childhood adversity, including neglect and abuse, has been identified as another risk factor for frequent attending in primary care (Reference Kapur, Hunt and MacFarlaneKapur 2004) and at medical out-patient departments in general hospitals (Reference Fiddler, Jackson and KapurFiddler 2004) and for somatoform disorders in adult life (Reference Borsini, Hepgul and MondelliBorsini 2014). However, childhood adversity is a risk factor for a variety of adverse physical and mental health outcomes in adult life (Reference ArnowArnow 2004).
Costs
It has been well established that patients with SESD use extraordinary amounts of healthcare resources, in both direct costs (e.g. out-patient physician visits) and indirect costs (e.g. early retirement and sickness absence) (Reference Konnopka, Kaufman and KonigKonnopka 2013). In the UK, Reference Kinder, Jorsh and JohnstonKinder et al (2006) reported a patient with a somatoform disorder whose costs to the National Health Service (NHS) were £209 000 in 2004 (not taking into account other potential costs such as primary care consultations, accident and emergency department visits, and attendances at other hospitals). It has also been shown that patients with MUS referred repeatedly to secondary care incur healthcare costs similar to those whose symptoms are explained by somatic disease (Reference Burton, McGorm and RichardsonBurton 2012a; Reference Anderson, Eplov and AndersenAndersen 2013).
Iatrogenesis and somatoform disorders
Patients with somatoform disorders are particularly likely to be subject to iatrogenic harm. There are many explanations for this. One reason is that they often misinterpret remarks/comments about their physical complaints, so that a reassuring comment from a neurologist that ‘We can't find anything seriously wrong on the scans’ is transformed in the patient's mind to ‘That's because you haven't looked hard enough’ or ‘You didn't find anything on the scan because I didn't have the headache on that day’. This may lead to seeking out further unnecessary tests. Other sources of iatrogenic harm derive from unwanted drug side-effects (e.g. morphine derivatives causing constipation) and inappropriate labelling of symptoms, for example ascribing a painful limb to complex regional pain syndrome when there is insufficient evidence to support this diagnosis and then arranging for an invasive treatment that leads to infection (Reference Kouyanou, Pither and WesselyKouyanou 1997).
In our clinical experience there are a number of rare medical syndromes that have high rates of coexisting functional disorders and use of opiates (often with unrecorded antecedent medical and psychiatric histories). Many of these patients bear a strong similarity to the polysyndromic phenotype described by Reference Warren, Clauew and WesselmanWarren et al (2014). These patients need to be screened for chronic somatoform disorders (Box 8).
BOX 8 Rare medical syndromes that share common characteristics with SESD
-
Sphincter of Oddi dysfunction type 3 (Reference Abraham, Anderson and LeeAbraham 1997)
-
Idiopathic slow-transit colon (Reference Dykes, Smilgin-Humphries and BassDykes 2001)
-
Loin pain haematuria syndrome (Reference Vakili, Alam and SollingerVakili 2014)
-
Complex regional pain syndrome type 1 (Reference Vranceanu, Barsky and RingVranceanu 2011)
-
Fowler's syndrome (Reference Hoeritzauer, Stone and FowlerHoeritzauer 2015)
The most serious iatrogenic complication is unnecessary surgery. Among women with interstitial cystitis or bladder pain, the number of previous functional syndromes such as fibromyalgia or irritable bowel syndrome was strongly correlated with the number of surgeries. This suggests that care should be taken before offering surgery to patients with a ‘polysyndromic phenotype’, characterised by repeated presentations of functional disorders over a long period of time (Reference Warren, Clauew and WesselmanWarren 2014) (Box 9).
BOX 9 Polysyndromic patient with multiple somatic syndromes
A 37-year-old woman was seen in the pain clinic with fibromyalgia or chronic widespread pain. She complained of pain all over her body since straining her left knee 2 years previously and was confined to a wheelchair and had an indwelling catheter. Her schooling had been interrupted by chronic abdominal pain and headaches and there was a history of childhood neglect with recurrent episodes of depression and self-harm. She had also suffered from irritable bowel syndrome, temporomandibular joint pains and urinary frequency since her mid-20s and had had dyspareunia and menorrhagia, leading to hysterectomy at the age of 29. Episodes of illness had led to frequent absences from work. All investigations (including bladder studies) were normal. She was being investigated for Fowler's syndrome, had a carer and was in receipt of disability-related financial support.
Identification: methods of assessment
It is clear from research findings that SESD often go unrecognised. The main reason for this is that patients often pursue their ‘illness journeys’ in non-psychiatric healthcare settings such as general hospital out-patient clinics. Another is that interviewing these patients is beset with difficulties. First, medical notes are not always available at the assessment interview; second, reported previous diagnoses by these patients (especially those with neurological symptoms) are often unreliable, especially if the list of previous diagnoses is particularly long (Reference Schrag, Brown and TrimbleSchrag 2004); and third, time is often restricted and does not allow for the collection of data from informants.
Electronic medical records
Recent use of electronic medical records to detect these patients in primary care has shown promise, and frequent attenders at out-patient clinics can be identified using computerised records (Reference den Boeft, van der Wouden and Rydell-Lexmondden Boeft 2014). Early detection in primary care would allow the general practitioner (GP) to engage with the patient proactively and develop a practical management plan. There is an urgent need to carry out more studies using this technology.
Documenting a chronology
Because of the constitutional nature of SESD (Reference Bass and MurphyBass 1995) we recommend the adoption of a developmental or life-course approach. This involves collecting all previous medical notes from both primary and secondary care and documenting a chronology such as the example in online Table DS1. This should be placed in the medical file.
Awareness among medical students
Identification would also be improved by increasing medical students’ awareness of SESD. Medical students are currently taught medicine and psychiatry as if they are separate disciplines; this ‘firewall’ does not reflect clinical realities and perpetuates the mind–body split that permeates our healthcare system (Box 4). Attitudes to teaching about somatoform disorders require a major shift, and should involve a role for both psychologists and primary care physicians, because it is incumbent on doctors in primary care (as well as specialist physicians) to identify patients with MUS at an earlier stage in the evolution of their illness, before secondary handicaps develop.
Problems in children
Up to half of paediatric primary care visits are for MUS (Reference van Ravesteijn, Wittkampf and Lucassenvan Ravesteijn 2009). A study of 161 patients admitted to a paediatric ward with diagnosed somatoform disorder revealed that the mean age was 14.4 years and 75% were female (Reference Bujoreanu, Randall and ThomsenBujoreanu 2014). The most common physical symptoms were pain (58%) and neurological symptoms (40%); 73% had a medical diagnosis and two-thirds a history of psychiatric treatment. Children with histories of trauma had significantly higher rates of psychiatric comorbidities and parental mental illness and were more likely to require in-patient psychiatric treatment on discharge (Reference Thomsen, Randall and IbeziakoThomsen 2014). Associations between consultation behaviour for MUS in children and parents has become a recent focus of investigation (Reference Shraim, Blagojevic-Bucknell and MallenShraim 2014).
Management
Where should it take place?
Addressing MUS is a priority for NHS England, and current plans involve delivering treatment via the Improving Access to Psychological Therapies (IAPT) programme (Improving Access to Psychological Therapies 2015). Recent emphasis has focused on the location of care. A number of initiatives have been introduced, including a symptom management clinic located within primary care (Reference Burton, Weller and MarsdenBurton 2012b; Reference Röhricht and ElanjitharaRöhricht 2014). Patients with complex somatoform disorders can be identified in primary care settings, where the involvement of the GP is considered crucial. A short feedback session with both GP and patient following the clinic helps to develop a collaborative approach to ongoing management. Early identification is important, as once SESD have become established, outcomes are poor.
What treatments are effective?
Because patients with SESD frequently use healthcare resources in a variety of settings, a key question about management involves not only where treatment is to take place, but also what type is offered and by whom.
Non-pharmacological interventions
A recent Cochrane review of non-pharmacological interventions for somatoform disorders (Reference Vakili, Alam and Sollingervan Dessel 2014) concluded that, compared with enhanced or structured care, psychological therapies showed no advantage but were generally superior to usual care or waiting list in terms of reduction in symptom severity, although effect sizes were small. Compared with enhanced care, cognitive–behavioural therapy (CBT) was not more effective. The authors drew attention to the fact that a substantial proportion of participants were not willing to accept psychological treatments.
A recent study of health anxiety in medical patients showed that a modified form of CBT was effective, and furthermore that those patients treated by non-mental health staff had better outcomes than those treated by other professional groups (Reference Tyrer, Tyrer and Lisseman-StonesTyrer 2015b).
Current evidence does not answer the question whether enhanced care delivered by primary care professionals has an effect on outcome. It may have an impact when delivered per protocol to well-defined groups of patients with functional disorders, but more intensive interventions are likely to be more successful in changing patient outcomes (Reference Rosendal, Blankenstein and MorrissRosendal 2013).
Tailored treatments
Because of the heterogeneity among patients with SESD (which overlap with patients with conversion disorder or functional neurological symptom disorder), treatments may have to be tailored to the type of clinical presentation (Box 10). For example, a patient with functional weakness in a wheelchair may require a different approach from a frequent attender at a gastroenterology clinic with multiple diverse symptoms. This key point needs to be considered in designing intervention studies for patients with SESD (Reference StoneStone 2014).
BOX 10 Overcoming psychosocial obstacles to recovery in SESD
A 19-year-old woman was referred by the neurology service with functional weakness in both legs and was confined to a wheelchair at home. Her house had been adapted and because of her immobility a home visit was organised. Although she denied any psychosocial problems at the initial assessment (apart from a history of severe irritable bowel syndrome) she agreed to be admitted to the neurology rehabilitation unit for further assessment. During this admission she revealed that her father was physically beating her and she had ‘retreated’ to her wheelchair to avoid further abuse (i.e. adopted an invalid role). This was discussed at a family meeting, after which her father left home and abandoned the family. This created a favourable environment for her to respond to both physiotherapy and psychological support. Following discharge she was well enough to pass her driving test, enrol in a local college and move to another city with her new partner.
There are useful websites for people with functional neurological disorders (e.g. www.neurosymptoms.org and www.nonepilepticattacks.info), and both patients and carers might benefit from them.
Service delivery and care management
It is clear that more research is needed to identify suitable service delivery pathways and type of service delivery that would provide adequate care for patients with SESD. A Dutch study proposed a stepped-care model for high-risk patients with chronic somatoform disorders and a problematic relationship with their GP: specialist multidisciplinary mental healthcare in a psychiatric setting with case management is recommended for this group (Reference Van der Feltz-Cornelis and Swinkelsvan der Feltz-Cornelis 2012). For more severe cases (e.g. patients confined to bed or a wheelchair), in-patient treatment may be required (Box 10). It is promising to note that commissioners have recognised that resources need to be assigned to this large, neglected and costly patient group (Reference Anderson, Eplov and AndersenAnderson 2013).
We therefore suggest the following approach to care management for SESD, consistent with the care programme approach:
-
regular monitoring of coexisting medical disorders
-
a multidisciplinary management plan reviewed every 6 months, possibly involving specialist (tier 3) personality disorder services (if personality disorder is present) and/or medical specialists
-
regular review of any benefits received (e.g. disability-related financial support and health aids); this may require liaison with community physiotherapists, occupational therapists, etc.
Management informed by attachment theory and disorders of early attachment
It has been shown that individuals with insecure adult attachment styles not only report more pain than those with secure attachment (Reference Davies, Macfarlane and McBethDavies 2009), but also are more likely to frequently attend primary care clinics with somatoform symptoms (Reference Taylor, Marshall and MannTaylor 2012). These observations suggest that understanding their behaviour as pathological help-seeking driven by difficulties in relating to caregiving figures may help doctors, especially those working in primary care, to see it in a different way and to adopt certain therapeutic strategies (Reference Adshead and GuthrieAdshead 2015).
For example, the finding that frequent attendance is associated with abnormal attachment style suggests that efforts should be made to keep this group of patients seeing the same GP, as there is a greater chance that a trusting relationship will develop than if they constantly see different doctors. Over time a therapeutic relationship can be developed in which underlying psychological and social problems may be revealed. There is also some empirical support (Reference Allen, Woolfolk and EscobarAllen 2006) for approaches that enable patients to receive regular care without it being contingent on symptom production. This might be achieved by (a) recognising that a patient wants support rather than another test and therefore directly addressing pathological health anxiety and worry; and (b) placing explicit boundaries on consulting behaviour by scheduling regular checks for reassurance rather than letting the patient drive the frequency of consultation.
Overlap with factitious disorders
DSM-5 suggests that factitious disorders should be considered as a variant of somatoform disorders (Reference Krahn, Bostwick and StonningtonKrahn 2008). There is some evidence of overlap in clinical practice, usually at the more severe end of the somatoform spectrum. Reference FinkFink (1992b) found that a fifth of ‘persistent somatisers’ had been admitted at least once for factitious illness, and in a study of women with fabricated or induced illness there was substantial co-occurrence of chronic somatoform and factitious disorder in two-thirds of the participants (Reference Bass and JonesBass 2011). Psychiatrists need to be alert to the impact of these illnesses on any dependent children.
Conclusions
It is important to identify patients with SESD because they often have severe mental illness and/or personality disorder and because of the associated high healthcare costs. Patients can present in a variety of medical settings and recent research suggests that identification and proactive management involving the GP in primary care may be the best way forward. Optimum management requires collaboration and communication between a variety of healthcare workers, but psychiatrists (especially those working in general hospitals) have a responsibility to identify and supervise the management of these patients, as well as to train others in current treatment approaches, while liaising with other health professionals and primary care clinicians. Healthcare commissioners need to be aware of the disability and financial burden posed by this patient group. Future research needs to concentrate on developing services to improve detection and adequate treatment of these patients. Innovative ways of engaging with patients in primary care settings need to be attempted, possibly with trained non-mental health staff.
MCQs
Select the single best option for each question stem
-
1 The prevalence of SESD in the general population is:
-
a 5–7%
-
b 20%
-
c 2%
-
d 50%
-
e 0.1%.
-
-
2 Patients with SESD satisfy the following criteria:
-
a drug misuse
-
b personality disorder
-
c repeated self-harm
-
d somatic symptoms that are distressing and persistent
-
e onset in adulthood.
-
-
3 Clinical features of SESD may not include:
-
a a chronic medical disorder
-
b a personality disorder
-
c lack of treatability
-
d abnormal illness behaviour
-
e abnormalities in the perception and reporting of physical symptoms.
-
-
4 Persistence in SESD is predicted by:
-
a absence of personality disorder
-
b number of physical symptoms
-
c number of primary care consultations
-
d involvement of family members
-
e age at onset.
-
-
5 Knowledge of attachment theory may be helpful in management because:
-
a of the presence of personality disorder
-
b sociopathic tendencies are common
-
c of problems with impulse control
-
d depersonalisation is common
-
e the consulting behaviour can be seen as a form of pathological help-seeking.
-
MCQ answers
| 1 | a | 2 | d | 3 | c | 4 | b | 5 | e |







eLetters
No eLetters have been published for this article.