LEARNING OBJECTIVES
-
Be aware that attention-deficit hyperactivity disorder (ADHD), anxiety disorders, depression and challenging behaviour (aggression and irritability) are frequently comorbid in ASD
-
Understand that current drug treatments mainly aim to ameliorate such comorbidities without targeting the biological mechanisms that are responsible for the core symptoms of ASD
-
Appreciate that for managing the core features of ASD, treatment programmes involving psychosocial, language and behavioural therapies remain the mainstay
In DSM-5 (American Psychiatric Association 2013), autism spectrum disorder (ASD) is now defined in a single category that previously included disorders that were considered separate such as pervasive developmental disorder (PDD), autistic disorder, Asperger syndrome, childhood disintegrative disorder and PDD not otherwise specified (PDD NOS), thus bringing all individuals with the same core symptoms into one designation. This description converges with the National Institute for Health and Care Excellence (NICE) guidelines that describe autism as ‘qualitative differences and impairments in reciprocal social interaction and social communication, combined with restricted interests and rigid and repetitive behaviours’ (NICE 2011). In August 2013, an evidence-based guideline was published that summarised the different ways that healthcare professionals can manage, treat and support children and young people with ASD (NICE 2013). In terms of specific intervention for the core features of ASD, this guideline indicated that antipsychotics, antidepressants, anticonvulsants or exclusion diets (such as gluten- or casein-free diets) should not be used to manage the core features in children and young people with autism (NICE 2013: recommendation 1.3.2).
ASD is frequently associated with comorbid disorders such as intellectual disability, anxiety disorder, attention-deficit hyperactivity disorder (ADHD), depression, obsessive–compulsive disorder (OCD) and tic disorder. From an epidemiological perspective, a review of studies published since 2000 suggests that the incidence of ASD is about 66/10 000, which equates to about 1/152 children, with males being affected more than females (at a ratio of 5:1) (Reference Hill, Zuckerman, Fombonne, Volkmar, Paul and RogersHill 2014).
Numerous studies have surmised that genetic predisposition and environmental factors might contribute to the pathophysiology of ASD (Reference Sandin, Lichtenstein and Kuja-HalkolaSandin 2014), but the precise mechanisms remain unknown. Nevertheless, evidence suggests that the deficits in social communication and comorbid symptoms can be managed by focused behavioural interventions, medication, and medication coupled with behavioural interventions. The Autism Diagnostic Observation Schedule (ADOS) can quantify impaired social communication and other ASD symptomatology, but few clinical trials have used it and shown it to capture clinically meaningful change.
In this article, we explore the treatment of ASD and its comorbidities in children and adolescents.
Treatment of ASD
Despite the advances made in the treatment of ASD, the treatment trajectory is hindered because specific biomarkers have not yet been identified for the classic ASD phenotypes such as autistic disorder or Asperger syndrome, making it more difficult to target the core symptoms pharmacologically. Clinically, the established practice is to target the symptoms of the comorbid conditions associated with ASD, such as hyperactivity, irritability, psychosis, depression, aggression and repetitive behaviour. Of note, evidence suggests an increased propensity for psychiatric comorbidities (including neurodevelopmental disorders) in individuals with ASD, with 70% of children with ASD having at least one comorbid psychiatric disorder and 41% having two or more (Reference Simonoff, Pickles and CharmanSimonoff 2008).
Although there are varying amounts of empirical evidence for targeted pharmacological interventions, a number of different drug types are routinely used to treat comorbidities associated with ASD in children and adolescents. These include, but are not limited to, antidepressants, antipsychotics, anticonvulsants and stimulants. Despite the extremely common use of these medications, there is a dearth of evaluable information to address their efficacy in treating the core symptoms of ASD.
Effective dosing with minimum side-effects
An important challenge for clinicians is to identify treatments that have a suitably balanced safety and efficacy profile. The ‘effective dose with minimum side-effects’ is the dose that achieves acceptable improvement of symptoms with minimum adverse effects (Reference SantoshSantosh 2014). Experience in the Centre for Interventional Paediatric Psychopharmacology and Rare Diseases (CIPPRD) has shown that medication should usually be initiated in small doses (typically an eighth to a sixth of the final anticipated dose), increasing about every five to six half-lives of the drug. In practice, for many drugs this is 3–7 days, with certain exceptions. It may require 4–6 weeks of titration to identify the most effective dose with minimum side-effects (Reference SantoshSantosh 2014). Fluoxetine liquid for anxiety or low mood, for example, might be initiated at 2–4 mg once a day, increased by 2–4 mg roughly every 7–8 days because of its long half-life (1–4 days) (Reference Altamura, Moro and PercudaniAltamura 1994) and increments made until the optimal dose is reached. This strategy needs to be discussed fully with the family and the patient. Feedback from the family to evaluate both the beneficial and adverse effects is vital (Reference SantoshSantosh 2014).
Recently, we began using HealthTracker™ (www.healthtracker.co.uk) Footnote a to monitor the profile of treatment response in children seen by the CIPPRD. This will allow longitudinal capture of lifetime response to different treatments, helping us to understand the effective dose with minimum side-effects and maximum therapeutic efficacy, taking into account symptom response, side-effects, impairment and global functioning, for each treatment. This leads to treatment-response profiling of patients and treatment optimisation. The HealthTracker system has been used in clinical and research settings to monitor medication response and side-effects, for example the multicentre Suicidality: Treatment Occurring in Paediatrics (STOP) study (www.stop-study.com) that longitudinally followed children and adolescents (many of whom had ASD) on fluoxetine, aripiprazole and risperidone.
Evaluation of the comorbidities in ASD
When planning treatment regimens, an important first step is to exclude underlying medical conditions, as it is quite common for changes in behaviour in ASD to result from a previously unrecognised physical condition causing pain or discomfort (Reference SantoshSantosh 2014), for example an unrecognised dental abscess. From clinical experience, a detailed physical examination ought to be undertaken in patients with ASD who have recently shown a change in behaviour. At the CIPPRD, identification and diagnoses of each co-occurring condition in ASD is made by confirming that the core symptoms of the co-occurring disorder are present (e.g. obsessions for diagnosing OCD); that symptoms reach ‘thresholds’ used in classificatory systems and are developmentally inappropriate; that symptoms are not ‘double counted’ (e.g. hyperactivity in ADHD and for hypomania); ensuring that each disorder produces additional impairment and checking that onset and course of the disorder are not indistinguishable and identical; and using multiple sources of information to ascertain the above criteria (Reference SantoshSantosh 2014).
Prescribing medications in ASD
Owing to the cognitive rigidity and sensory problems encountered in patients with ASD, adapting prescribing to suit individuals is an important factor to consider in the management of treatment. This is described in more detail elsewhere (Reference SantoshSantosh 2014). Individuals with ASD may not take a medication because of its odour, taste or formulation, so these factors should be carefully considered and managed for each patient.
An example of medication decision-making for comorbidity associated with ASD is presented in Table 1 and the evaluable evidence for this decision-making scheme can be deduced from the literature described in the following sections. Table 1 is intended to be used only as a general guide and is based on clinical experience (taking both efficacy and adverse events into account) and not on empirical evidence.
TABLE 1 Example of a medication decision-making schema for a child with autism spectrum disorder and comorbid disordersa
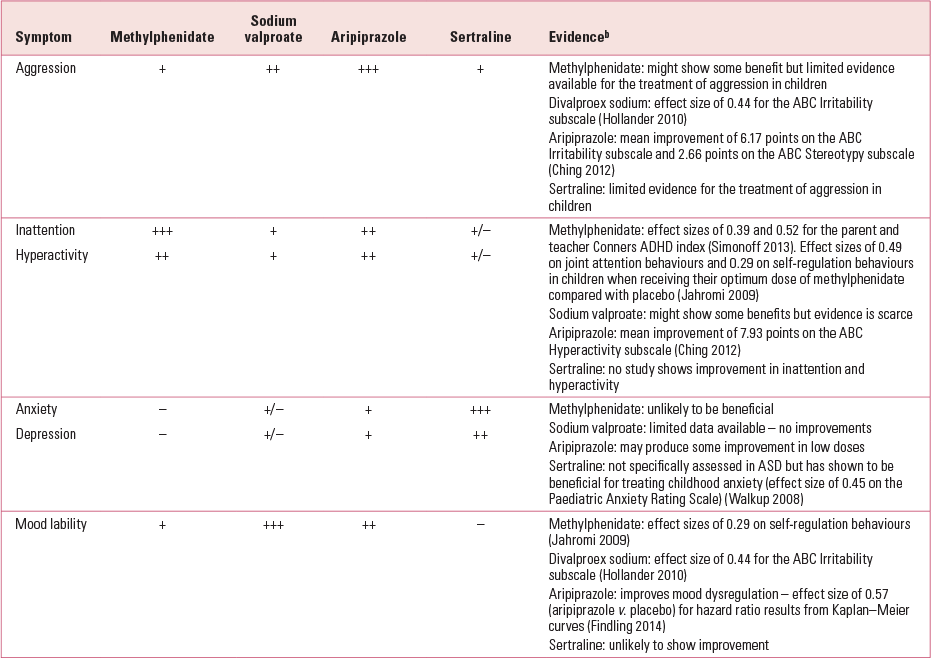
Holistic multimodal treatments are essential for the management of ASD. Treatment programmes such as psychosocial, language and behavioural therapies are not covered in this review, but it would be necessary to use them in conjunction with medication.
Treatments for aggression and irritability
Perhaps the most frequent and detrimental comorbidity associated with ASD is aggression. Typical and atypical antipsychotics have been used to treat it and the salient points will be described here.
Risperidone
In one randomised double-blind clinical trial (carried out by the Research Units of Paediatric Psychopharmacology (RUPP) Autism Network) involving 101 children with autistic disorder (mean age 8.8 years, s.d. = 2.7), risperidone (dose range 0.5–3.5 mg/day, mean 1.8 mg/day, s.d. = 0.7) was safe, well tolerated and efficacious for the treatment of tantrums, aggression and self-injurious behaviour (Reference McCracken, McGough and ShahMcCracken 2002). The primary outcome measures were the Irritability subscale scores of the Aberrant Behavior Checklist (ABC) and the Clinical Global Impressions – Improvement (CGI-I) scale at 8 weeks. However, a follow-up study did not find significant improvement in core symptoms such as social interaction and communication as assessed by change in scores on the Children's Yale–Brown Obsessive Compulsive Scale, the Ritvo–Freeman Real Life Rating Scale and the Maladaptive Behaviour domain of the Vineland Adaptive Behavior Scales (Reference McDougle, Scahill and AmanMcDougle 2005).
In another study, based on ratings on the Childhood Autism Rating Scale (CARS) and Children's Global Assessment Scale (CGAS), risperidone was effective in improving global functioning and social responsiveness, and lessening hyperactivity and aggression in children with autism as young as 2 years of age (Reference Nagaraj, Singhi and MalhiNagaraj 2006).
A Cochrane review (Reference Jesner, Aref-Adib and CorenJesner 2007) showed some evidence of the benefits of risperidone in treating irritability, repetitive behaviours and social withdrawal in ASD. The authors stressed, however, that the findings from the review should be treated with caution as small sample sizes and the lack of a standardised outcome measure preclude the making of meaningful inferences.
Aripiprazole
Aripiprazole at dose ranges of 5–15 mg/day for 8 weeks has been reported to be efficacious, safe and well tolerated for treating irritability in children and adolescents with autism who were presenting with tantrums, aggression or self-injurious behaviour (Reference Marcus, Owen and KamenMarcus 2009; Reference Owen, Sikich and MarcusOwen 2009).
A Cochrane review (Reference Ching and PringsheimChing 2012) showed a mean improvement of 6.17 points on the ABC irritability subscale, 7.93 points on the hyperactivity subscale and 2.66 points on the stereotypy subscale in children administered aripiprazole in comparison to those on placebo. The authors also noted that lengthier studies are warranted to garner information on safety, tolerability and efficacy of aripiprazole in the long term.
More recently, Findling et al (2014) evaluated the safety and efficacy of aripiprazole compared with placebo in preventing relapse of irritability associated with ASD in 85 patients (41 on aripiprazole, 44 on placebo) aged 6–17 years old. In this relapse-prevention trial, the difference between aripiprazole and placebo in time to relapse was not statistically significant during maintenance treatment. However, during maintenance therapy some secondary efficacy measures were statistically significant in comparison with placebo for patients treated with aripiprazole.
Risperidone and aripiprazole
Both risperidone and aripiprazole have been approved by the US Food and Drug Administration to treat irritability associated with ASD. However, as far as we are aware, only one study has been published that makes a direct head-to-head comparison between the two drugs (Reference Ghanizadeh, Sahraeizadeh and BerkGhanizadeh 2014). It used change in ABC scores as the primary outcome measure, and the findings showed that the efficacy, along with the safety and tolerability profiles, of aripiprazole and risperidone were comparable.
Other atypical antipsychotics
An 8-week, double-blind placebo-controlled trial of olanzapine, involving eight patients diagnosed with PDD, six with autism, one with Asperger syndrome and four with PDD NOS, showed a 50% response in CGI-I score with olanzapine in comparison to placebo (20% of responders) (Reference Hollander, Wasserman and SwansonHollander 2006a).
Studies using quetiapine (Reference Hardan, Jou and HandenHardan 2005; Reference Golubchik, Sever and WeizmanGolubchik 2011), ziprasidone (Reference Dominick, Wink and McDougleDominick 2015) and clozapine (Reference Zuddas, Ledda and FrattaZuddas 1996; Reference Gobbi and PulvirentiGobbi 2001) have shown some promise in improving irritability and hyperactivity. However, these findings should be treated with caution as small sample sizes and open-label design preclude definitive inferences from being made. More work is needed to demonstrate the efficacy of these drugs in the clinical environment.
Metabolic syndrome
There is a strong correlation between metabolic syndrome (weight gain, dyslipidaemia and type II diabetes) and the use of antipsychotics in young people (Reference NewcomerNewcomer 2005). Almandil et al (2013) showed that olanzapine, risperidone and aripiprazole were all associated with weight gain in children and adolescents. Moreover, Bobo et al (2013) indicated that children and adolescents prescribed antipsychotics had a threefold increased risk for type II diabetes. As the safety and tolerability profile of each atypical antipsychotic is specific, it is recommended that adverse events associated with metabolic syndrome should be carefully monitored to inform treatment strategies. Despite this, to date there are no clinically validated monitoring mechanisms in place. Internet-based monitoring of side-effects and remote physiological monitoring of cardiac function using wearable technology needs exploration because of the long-term negative impact of psychotropic-induced weight gain and metabolic syndrome.
Benzodiazepines
Owing to their propensity to induce tolerance and the adverse events associated with them, benzodiazepines are not recommended for the management of ASD, especially over the long term. They are sometimes used in acute situations to calm aggression, but disinhibition can occur, which can exacerbate the symptoms (Reference SantoshSantosh 2014).
Treatment for inattention and hyperactivity
Although ASD presents with myriad symptoms, inattention and hyperactivity are frequently encountered in children with the disorder and often warrant a diagnosis of ADHD (Reference Santosh and ColonneliSantosh 2012; Reference Lee, Oh and ParkLee 2014). Refer to the review by Reference Santosh and ColonneliSantosh & Colonneli (2012) for detailed information on the comorbidity of ASD and ADHD and its treatments.
Methylphenidate
A large open-label clinical study comparing stimulant treatment (mainly methylphenidate) of a group of children and adolescents with ADHD and a group with ADHD comorbid with ASD found good symptom reduction in both groups (Reference Santosh, Baird and PityaratstianSantosh 2006). In a large double-blind placebo-controlled trial (122 children aged 7–15 years with intellectual disability and hyperactivity) that used the parent/teacher ADHD index of the Conners Rating Scale – Short Version at 16 weeks as the primary outcome measure, optimal dosing with methylphenidate was effective in some children (Reference Simonoff, Taylor and BairdSimonoff 2013).
A secondary analysis of RUPP Autism Network data showed that methylphenidate had positive outcomes in social communication and self-regulation in 33 children with PDD and hyperactivity (29 boys and 4 girls, aged 5–13 years) (Reference Jahromi, Kasari and McCrackenJahromi 2009). These data suggest that methylphenidate dosing is related to changes in joint attention (modulating social behaviours), with lower doses being better than higher doses. It is likely that higher doses are necessary if the target is hyperactivity.
Amphetamines
Amphetamines have been used for the treatment of ADHD in children; however, there are no published studies on the efficacy of amphetamines in children and adolescents with ASD. Lisdexamfetamine dimesylate is a therapeutically inactive pro-drug comprising of d-amphetamine bound to the amino acid lysine. Using the investigator-rated ADHD Rating Scale IV (ADHD-RS-IV) as the primary outcome measure, lisdexamfetamine dimesylate was efficacious for the treatment of ADHD in children and adolescents aged 6–17 years and was well tolerated (Reference Coghill, Banaschewski and LecendreuxCoghill 2013). Lisdexamfetamine dimesylate may be an agent that could be considered for managing ADHD in ASD.
Atomoxetine
In a comprehensive review examining the effects of atomoxetine in young people with developmental disabilities, Aman et al (2014) reported a positive outcome of atomoxetine on ADHD in all ten studies that were evaluated. However, only two of these were randomised double-blind placebo-controlled trials that focused on children with ASD. One (Reference Arnold, Aman and CookArnold 2006) had a small sample (n = 16), and although the other (Reference Harfterkamp, van de Loo-Neus and MinderaaHarfterkamp 2012) had a much larger sample (n = 102), it used only parent and clinician ratings as outcome indicators (Reference Aman, Smith and ArnoldAman 2014).
Using parent-based outcome measures (the ABC and the Children's Social Behaviour Questionnaire (CSBQ)), a randomised double-blind placebo-controlled 8-week trial in 97 patients with ASD and ADHD recently showed that atomoxetine had no effect on core ASD symptoms (Reference Harfterkamp, Buitelaar and MinderaaHarfterkamp 2014). More recently, another study showed no evidence for predictors of response to atomoxetine in children and adolescents with ASD and ADHD symptoms (Reference Harfterkamp, van der Meer and van der Loo-NeusHarfterkamp 2015).
Alpha-2 adrenergic receptor agonists
Clonidine and guanfacine are alpha-2 adrenergic agonists that have shown some promise in treating ADHD in ASD. In one double-blind placebo crossover study involving nine males with autism aged 5–33 years, transdermal clonidine was effective in ameliorating hyperactivity (Reference Fankhauser, Karumanchi and GermanFankhauser 1992). However, others have argued that clonidine is unlikely to be of benefit for the treatment of ADHD in individuals with ASD (Reference HazellHazell 2007). Sedation is a frequent adverse effect of clonidine and, in comparison, guanfacine might have a better safety and tolerability profile. Guanfacine is thought to be less sedating and its half-life is longer (10–30 h) than that of clonidine (4–10 h), which might lead to an easier dosing regimen. Open-label studies suggest some improvements in hyperactivity and impulsiveness and, to a lesser extent, in teacher-reported outcomes (Reference Scahill, Aman and McDougleScahill 2006). More recently, in a multisite randomised placebo controlled trial involving 62 children with ASD (mean age of 8.5 years), extended-release guanfacine was shown to be safe, well-tolerated and efficacious in lowering hyperactivity, distractibility and impulsivity (Reference Scahill, McCracken and KingScahill 2015).
Treatment for anxiety and depression
Anxiety disorders frequently present in children and adolescents with ASD, with comorbidity ranges of 40–84% for any anxiety disorder, 8–63% for specific phobias, 5–23% for generalised anxiety, 13–29% for social anxiety and 8–27% for separation anxiety (Reference Sukhodolsky, Bloch and PanzaSukhodolsky 2013). The usual treatment for anxiety in ASD involves selective serotonin reuptake inhibitors (SSRIs), including sertraline, fluoxetine, fluvoxamine and citalopram. These SSRIs have all demonstrated efficacy in children with anxiety disorder and, although they have not specifically assessed in ASD, sertraline together with cognitive–behavioural therapy has proved beneficial for the treatment of childhood anxiety (Reference Walkup, Albano and PiacentiniWalkup 2008).
Another agent that has targeted anxiety in children with ASD and shows some effectiveness is buspirone (Reference Buitelaar, van der Gaag and van der HoevenBuitelaar 1998). More recently, the classical beta-adrenergic antagonist propranolol has shown positive cognitive effects in the brains of adults with ASD (Reference Narayanan, White and SaklayenNarayanan 2010); however, more work is needed to properly evaluate its use for the treatment of anxiety in children with ASD.
There is limited evidence for the effectiveness of SSRIs as a treatment for depression in children with ASD (Reference Williams, Brignell and RandallWilliams 2013). However, the use of fluoxetine can be warranted in a child with ASD who is severely depressed (Reference SantoshSantosh 2014). In general, clinical decision-making regarding the treatment of depression in children and adolescents with ASD should be made on a case-by-case basis.
Treatment for sleep disorders
Sleep disorders are common in children with ASD. Between 40 and 80% of children experience some type of sleep disorder in comparison with 25–40% in typically developing children. The most common sleep disorders are insomnia, reduced sleep efficiency, bedtime resistance and daytime sleepiness. A comprehensive meta-analysis evaluating melatonin in the treatment of sleep disorders in ASD (Reference Rossignol and FryeRossignol 2011) showed that it was well tolerated and might improve sleep parameters and daytime behaviours.
A randomised placebo-controlled Phase III trial evaluated the efficacy of melatonin for sleep problems in 146 children (age 3–15.8 years) with neurodevelopmental disorders (Reference Gringras, Gamble and JonesGringras 2012). Using total sleep time at night after 12 weeks (adjusted for baseline) recorded in sleep diaries by parents as the main outcome measure, it showed some improvements in the child's sleep with melatonin (i.e. children fell asleep more quickly), but the child's behaviour and family functioning measures did not significantly improve.
Treatment of mood lability and bipolar disorder
Anti-epileptic drugs are used for bipolar disorder and mood lability in children with ASD, and they have also been prescribed for impulsivity, irritability, aggressive behaviour and rage. In a randomised double-blind placebo-controlled trial evaluating the efficacy of divalproex sodium for the treatment of irritability in 55 children with ASD using the ABC and CGI-I Irritability subscales as primary outcome measures, statistically significant improvements in irritability and aggression were reported (Reference Hollander, Chaplin and SooryaHollander 2010). Other evidence suggests that aripiprazole and risperidone are beneficial in improving mood dysregulation (Reference McCracken, McGough and ShahMcCracken 2002; Reference Findling, Mankoski and TimkoFindling 2014).
Treatment of social disability, repetitive behaviours and self-injurious behaviour
Social disability
An overview of the treatment of social disability is presented elsewhere (Reference SantoshSantosh 2014). One way in which social disability can be measured in children with ASD is by using the parent-rated Social Withdrawal subscale of the ABC. This subscale monitors social disability by capturing recognisable behaviours in response to interaction instigated by others and the degree to which the child begins the interaction (Reference Scahill, Hallett and AmanScahill 2013). In a secondary analysis on ABC Social Withdrawal subscale data from two multicentre trials, it was proposed that after 8 weeks of treatment, risperidone was effective for the treatment of social disability in children with ASD (Reference Scahill, Hallett and AmanScahill 2013).
Repetitive behaviours and circumscribed interests
Although some studies have suggested that SSRIs might help in ameliorating repetitive behaviours and circumscribed interests in children and adolescents with ASD, a Cochrane review examining nine randomised controlled trials with a total of 320 participants showed that there was no evidence to demonstrate that SSRIs (fluoxetine, fluvoxamine, fenfluramine or citalopram) were beneficial in comparison to placebo (Reference Williams, Brignell and RandallWilliams 2013). SSRIs can often cause behavioural activation when used in ASD.
In an 8-week double-blind placebo-controlled trial, Reference Hollander, Soorya and WassermanHollander et al (2006b) showed that divalproex sodium might be beneficial in improving repetitive behaviours in individuals with ASD.
Self-injurious behavior
Self-injurious behaviour presents one of the most challenging behavioural disorders in ASD and is particularly distressing for parents. Such behaviour can include, but is not limited to, hand biting, hitting the head against objects or hands, and skin scratching.
It has been reported that risperidone is effective in targeting self-injurious behaviour, especially in severe cases (Reference McCracken, McGough and ShahMcCracken 2002). Opioid antagonists such as naltrexone have been used for the management of self-injurious behaviour based on the premise that there is an abnormality in pain control in individuals with ASD with these behaviours. Although open-label studies suggest that naltrexone might be beneficial for children with self-injurious behaviour and autism (Reference Elchaar, Maisch and AugustoElchaar 2006), double-blind placebo-controlled clinical trials found it to be no different than placebo for managing these behaviours (Reference Willemsen-Swinkels, Buitelaar and NijhofWillemsen-Swinkels 1995).
A summary of the common comorbidities in ASD and their treatment is presented in Table 2.
TABLE 2 Common comorbidities in autism spectrum disorder and their treatmenta

Disorder-modifying treatments
Comorbidity associated with ASD frequently compounds the treatment of core symptoms observed in the disorder. Hitherto no validated pharmacological interventions that directly aim to ameliorate the core social and communication defects of ASD have been available. In recent years a paradigm shift in the understanding of the pathophysiology of ASD has allowed new candidates to be identified that have the potential to act as disorder-modifying agents. The pertinent observations are described in this section.
Sulforaphane
Sulforaphane, a compound found in cruciferous vegetables, up-regulates antioxidant genes involved in control mechanisms through the Keap1-Nrf2 cytoprotective signalling pathway. This leads to modulation of oxidative stress, antioxidant capacity and neuroinflammation. Sulforaphane has recently been evaluated in a randomised double-blind placebo-controlled trial involving young males (n = 44; 29 on active treatment, 15 on placebo) aged 13–27 years with moderate to severe ASD (Reference Singh, Connors and MacklinSingh 2014). This showed that patients receiving daily doses of 50–150 μmol sulforaphane for 18 weeks had statistically significant improvements in social interaction, irritability, hyperactivity and verbal communication scores in comparison with patients receiving placebo (as assessed by change on ABC Irritability subscale, Social Responsiveness Scale (SRS) and CGI-I scores). Further evaluation in larger-scale clinical trials will be required to confirm the efficacy of sulforaphane in ASD.
Oxytocin
Oxytocin is a hormone comprising nine amino acids made in the hypothalamus and secreted from the posterior pituitary gland. It has been surmised that oxytocin plays a key role in social affiliation and attachment, and that variation in the oxytocin receptor gene might be responsible for ASD (Reference Wermter, Kamp-Becker and HesseWermter 2010). A randomised double-blind placebo-controlled trial evaluating the efficacy of intranasal oxytocin, using change in caregiver-completed SRS and clinician-rated CGI-I scores as primary outcome measures in 50 males with ASD aged 12–18 years, showed that the primary and secondary efficacy outcome measures in active treatment (n = 26) were no different to placebo (n = 24) (Reference Guastella, Gray and RinehartGuastella 2015).
In another randomised double-blind placebo-controlled trial, intranasal oxytocin (24 international units (IU), administered on two visits separated by 1 week) was tested on 32 adult males with ASD and 32 placebo controls using Tobii eye tracking to determine whether it could improve eye gaze behaviour, a key marker for social communication difficulties in ASD (Reference Auyeung, Lombardo and HeinrichsAuyeung 2015). This study showed that intranasal oxytocin increased eye gaze behaviour in both the autism and placebo groups, with patients in the autism group who spent less time looking to the eye region benefiting most from intranasal oxytocin treatment.
N-acetylcysteine
N -acetylcysteine is a prodrug of L-cysteine and following oral administration is rapidly absorbed. Subsequently, the uptake of cystine results in the shuttling of glutamate out of the cell and is therefore pivotal in the regulation of extracellular glutamate levels. This is important because it has been recognised that dysregulation of glutamatergic neurotransmission and glutathione synthesis might be causative factors in ASD. A randomised placebo-controlled trial has provided evidence for the efficacy of N-acetylcysteine in treating irritability in children with autism (Reference Hardan, Fung and LiboveHardan 2012). Recently, a randomised double-blind placebo-controlled trial has shown that N-acetylcysteine is an effective adjunct to risperidone for the treatment of irritability in children 4–12 years of age with autism (Reference Nikoo, Radnia and FarokhniaNikoo 2015). Although promising, further work is required to determine a role for N-acetylcysteine in the treatment of ASD.
d-cycloserine
Originally known for its use as an effective antibiotic for the treatment of pulmonary tuberculosis (Reference Walker and MurdochWalker 1957), d-cycloserine has gained attention for its potential use as a treatment in psychiatric disorders (Reference Schade and PaulusSchade 2015). d-cycloserine is a glycine partial agonist at the N -methyl-d-aspartate (NMDA) receptor, and a pilot study of its use showed significant improvements in social withdrawal in individuals with autism (Reference Posey, Kem and SwiezyPosey 2004). More recently, others have observed positive effects of d-cycloserine on stereotypies (Reference Urbano, Okwara and ManserUrbano 2014) and social deficits (Reference Urbano, Okwara and ManserUrbano 2015) in individuals 14–25 years old with ASD. These data suggest that d-cycloserine might be useful in ameliorating core deficits of ASD; however, larger double-blind placebo-controlled studies would be required to further develop and expand on these results.
Insulin growth factor 1
It has been hypothesised that brain development is altered in ASD, and crucially insulin growth factor 1 (IGF-1) is important for brain development. Elevated levels of IGF-1 and other growth factors have been found in boys with ASD (Reference Mills, Hediger and MolloyMills 2007). Recently, a Phase I study evaluating the safety, pharmacokinetics and efficacy of recombinant human IGF-1 (mecasermin) in 12 girls aged 2–10 years with Rett syndrome showed a mixed clinical picture. SPreliminary efficacy evaluations on neurobehavioral parameters produced a mixed picture: on some measures some girls showed improvements, whereas others showed deterioration (Reference Khwaja, Ho and BarnesKhwaja 2014). Further work is warranted to evaluate the efficacy of IGF-1 in children with Rett syndrome.
Complementary and alternative medicines
Studies on complementary and alternative medicines (CAMs) for the treatment of ASD and its comorbidities lack empirical scientific evidence and therefore limited information is available to ascertain whether they have any impact on the comorbidities associated with ASD. Reference Levy and HymanLevy & Hyman (2008) have suggested that 50–70% of children with ASD in treatment studies receive CAMs. Frequently used therapies include chelating agents, vitamins and essential fatty acids.
Many children with autism have received chelation therapy, but there is considerable circumspection with regard to its efficacy in ASD (Reference BrentBrent 2013). Patients given chelation therapy can experience serious adverse events, and previously the US Food and Drug Administration has indicated that promoting the use of over-the-counter chelation products for the treatment of autism and other conditions was deemed to be unsafe and illegal (Reference VoelkerVoelker 2010).
Perhaps the most widely used CAMs are vitamin supplements and essential fatty acids. One review reports that at present there is no evidence to indicate a role for omega-3 fatty acids in the treatment of autism (Reference Williams and MarraffaWilliams 2012).
Future perspectives
The ultimate goal of drug intervention in ASD is to improve the quality of life of patients by targeting the core symptoms of the disorder, rather than just its comorbidities. As far as we are aware, there is no evidence to indicate a treatment strategy that has a long-lasting impact on ASD and brings about recovery. Although research efforts to develop agents to ameliorate the core symptoms of ASD have intensified in recent years, these studies are limited in their sample size and much larger clinical trials of sufficient power are warranted to tease out the pertinent information. In the meantime, comprehensive treatment programmes such as psychosocial, language and behavioural therapies, coupled with drug treatments with careful monitoring of symptoms and adverse events, allow clinicians and healthcare workers in child and adolescent mental health services to make significant improvements in the quality of life of young people with ASD.
MCQs
Select the single best option for each question stem
-
1 The 2013 NICE evidence-based guidelines on the management and support of children and young people with ASD advise that:
-
a antipsychotics can be used to manage the core features of autism in children and young people
-
b antipsychotics and antidepressants can both be used to manage the symptoms of autism in children and young people
-
c exclusion diets (e.g. gluten- or casein-free diets) can be used to manage the core features of autism in children and young people
-
d antidepressants can be used to manage the repetitive behaviours and circumscribed interests of autism in children and young people
-
e antipsychotics, antidepressants, anticonvulsants or exclusion diets (e.g. gluten-or casein-free diets) should not be used to manage the core features of autism in children and young people.
-
-
2 SSRIs:
-
a help to reduce mood lability in ASD
-
b are not useful in reducing repetitive behaviour and circumscribed interests in ASD
-
c have proven efficacy in managing anxiety in ASD
-
d rarely produce behavioural activation in ASD
-
e frequently help reduce hyperactivity in ASD.
-
-
3 The best drug choice for treating severe aggression in children with ASD is:
-
a sodium valproate
-
b methylphenidate and sodium valproate
-
c aripiprazole
-
d methylphenidate
-
e sertraline.
-
-
4 The best drug choice for treating inattention, impulsivity and hyperactivity (ADHD) in children with ASD is:
-
a methylphenidate and aripiprazole
-
b methylphenidate or atomoxetine
-
c aripiprazole
-
d sertraline
-
e sodium valproate.
-
-
5 A potential disorder-modifying agent for ASD is:
-
a clonidine
-
b risperidone
-
c aripiprazole
-
d fluoxetine
-
e N-acetylcysteine.
-
MCQ answers

| 1 | e | 2 | b | 3 | c | 4 | b | 5 | e |








eLetters
No eLetters have been published for this article.