There are more guidelines for the treatment of depression than for any other psychiatric disorder. The latest versions emphasise their evidence-based nature and, in the UK, the British Association for Psychopharmacology (BAP) has updated its guidelines on the treatment of depression with antidepressants (Reference Anderson, Nutt and DeakinAnderson et al, 2000). Other substantial guidelines include the revised American Psychiatric Association practice guidelines (American Psychiatric Association, 2000), which are probably the best known, those of the Canadian Psychiatric Association (Canadian Psychiatric Association, the Canadian Network for Mood and Anxiety Treatments (CANMAT), 2001) and from the World Federation of Societies for Biological Psychiatry (Reference Bauer, Whybrow and AngstBauer et al, 2002). The National Institute for Clinical Excellence (NICE) is also developing depression guidelines for England and Wales. Each of these sets of guidelines takes a slightly different perspective but they all depend on the same evidence base, although they use slightly different systems of evidence-grading and methods for linking this to recommendations. In general, and reassuringly, the guidelines vary relatively little in their core messages but tend to minimise uncertainties and gloss over areas of difficulty. I include in this the BAP guidelines in which I was involved, but would direct you to them for a general review of the evidence (Reference Anderson, Nutt and DeakinAnderson et al, 2000).
Mindful of the wealth of information already available and the vast potential scope, I will discuss a few important areas here that are given little emphasis in most reviews.
The nature of evidence
Evidence is usually graded using quality criteria based on the pre-eminence, at least for treatment studies, of the double-blind randomised controlled trial (RCT) designed to minimise biased outcomes. The highest-quality evidence is therefore from an appropriate systematic review and meta-analysis of good-quality RCTs, with lower ranking being given to ‘weaker’ experimental designs such as non-randomised studies, open trials or case reports. At the lowest level is clinical ‘expert’ opinion or observation (Box 1).
Reporting bias aside, the level of evidence required to be convincing about an outcome is dependent on how large the effect is, how prone it is to being influenced and how likely it is to have occurred by chance. For example, hard outcomes such as death have little ascertainment bias, whereas rating scale scores, the primary outcomes in most psychiatric research, are very vulnerable to measurement bias. However, even the gold-standard RCT has caveats, at least in its interpretation (Box 1). The first is that an RCT can answer only the question it addresses and study designs can be manipulated to emphasise certain outcomes over others. Commercial drug study designs will therefore tend to lean towards the strengths of the compound in which the sponsor is interested and the weaknesses of comparator treatments; in some cases, certain analyses and lines of investigation may not be pursued. The reporting of results is also prone to a similar ‘spin’ in emphasis. Much of the data from comparative drug trials in depression falls into this area of concern.
Box 1 General principles for assessing quality of intervention studies
High quality: systematic reviews of good-quality randomised controlled trials (RCTs) or a large definitive RCT
Intermediate quality: small RCTs, non-randomised or open studies
Low quality: case reports, opinion
RCT caveats
Designs may be biased or selected to achieve specific aims
There may be ‘spin’ in reporting results or a bias in publication
Heterogeneity within groups may be concealed
There may be a lack of generalisabilty or clinical practice relevance
A second issue is reproducibility and the management of conflicting outcomes and results of small studies, which may throw up findings by chance or fail to find differences. Here it is important take results not at their face value but only as bricks in the wall of evidence. This category usually includes evidence relating to what is effective for treatment-resistant depression or where the first treatment fails.
Another problem for the RCT is that of heterogeneity and the other side of the coin, generalisability. The RCT gives a group outcome only for the subjects who are entered into it and this group may be an unrepresentative minority of those treated in clinical practice (Reference Zimmerman and PosternakZimmerman & Posternak, 2002).
Randomised controlled trials are important for determining efficacy (whether something really does work) but they are weaker at indicating effectiveness (whether the treatment works in usual practice) and very weak at identifying small subgroups which might benefit from or be harmed by an intervention. This is partly a matter of numbers and is, of course, the reason for post-marketing surveillance for rare adverse effects. It also applies to the current controversy as to whether or not selective serotonin reuptake inhibitors (SSRIs) promote suicidal activity in a small minority of patients.
The issue of the generalisability of an RCT is of vital importance for policy determinations. However, it is one of the weaker areas of evidence because of the complexity of such studies and the methodological problems inherent in undertaking them. It is relevant, for example, to the issue of when to use antidepressants in primary care practice, given uncertainties about the severity threshold above which patients are likely to get benefit.
When are antidepressants an effective choice?
The evidence that antidepressants are more effective than placebo in the treatment of major depressive disorder needs little rehearsal (although for a critical view, see Reference MoncrieffMoncrieff, 2002). It is worth a moment's reflection on what this means with issues centred on the size of effect and clinical relevance. Evidence from meta-analyses indicates that in short-term trials, generally of 4–12 weeks, about 50–60% of patients with depression ‘respond’ to antidepressants compared with about 30% to placebo, giving a number needed to treat (NNT) of 4–5. Although methodologies differ, these figures tend to assign those that drop out not accounted for elsewhere to treatment failures. Therefore, the responses rates and advantage to antidepressants are likely to be higher, certainly in those complying with treatment.
It is also important to recognise that response to placebo is not the same as having no treatment. Rather, specific pharmacological response is being teased out from the patient's response to non-pharmacological factors. Response is usually defined as a 50% reduction in a rating scale score (typically the Hamilton Rating Scale for Depression, HRSD) or ‘much improved’ or ‘very much improved’ on the Clinical Global Impression Scale. However, it is recognised increasingly that many people continue to have significant symptoms and that the stricter definition of remission (e.g. below an absolute score such as an HRSD less than 9) reveals a more disappointing rate of about 40% compared with 25% for placebo (Reference Thase, Entsuah and RudolphThase et al, 2001; Reference Smith, Dempster and GlanvilleSmith et al, 2002), giving an NNT of 6–7.
Is this generally true of all depressive disorders which, of course, cover more than major depressive disorder? Putting aside the debate about different types of depression, it is possible to characterise depressive disorders very roughly in terms of severity and duration and I believe that these tend not to receive the consideration they deserve. The distinction between major depressive disorder and milder depression, often called ‘minor’ depression, is based on severity, in terms of number of symptoms and the magnitude of each. The distinction is one with a rough-and-ready threshold. This has consequences for management as it is often taken as a cut-off for the need for specific treatment. A major concern, and one that fuelled the Defeat Depression Campaign in the UK, has been about adequate detection of depression (major depressive disorder) in primary care as the first step in providing more effective treatment. However, this crucially begs the question about when, and to what extent, antidepressants are effective for the severity of depression predominantly seen in primary care.
Severity of depression and response to antidepressants
It is difficult to determine the threshold of severity of depression at which it is possible to demonstrate the efficacy of antidepressants over placebo. Most antidepressant studies involving placebo use a minimum HRSD score of 17 or 18, which is appropriate for secondary care and identifies those who at least have moderately severe major depressive disorder. However, patients seen in primary care are, on average, more mildly depressed and studies show mean depression scores in the 13–16 range. At these levels, it is very difficult to distinguish between minor depression and mild major depressive disorder.
In a post hoc analysis of a study in primary care, Reference Paykel, Freeling and HollymanPaykel et al(1988) showed that patients with milder depression – either defined as minor depression or those with HRSD scores below 13 – did not benefit from antidepressants over placebo. Reference OttevangerOttevanger (1991) found a higher HRSD threshold of 20 and similar thresholds have been seen in other studies. A recent analysis of the Food and Drug Administration database including only data from studies with HRSD scores of 23 and above found that the superiority of antidepressants over placebo became more likely as severity of depression increased; also, the degree of improvement in HRSD scores with placebo treatment tended to diminish with severity whereas the opposite was true with antidepressants (Reference Khan, Leventhal and KhanKhan et al, 2002). Indeed, the evidence for benefit from antidepressants was equivocal at initial HRSD scores of 23–24.
Studies concentrating on patients with minor depression (i.e. short-term depression not meeting criteria for major depressive disorder) do not, in general, find that antidepressants are better than placebo (e.g. Reference Barrett, Williams and OxmanBarrett et al, 2001) and even where a statistical advantage is present, the clinical importance is uncertain. These findings are supported by more naturalistic intervention data showing that improving adequacy of treatment for minor depression does not alter outcome (e.g. Reference Peveler, George and KinmonthPeveler et al, 1999). What appears to account for the lack of advantage that antidepressants show in treatment of milder depressions is the high placebo response rate. In other words, milder, acute-onset depressions have a high likelihood of resolving, which should not surprise us.
The picture appears different, however, for dysthymia (milder depression that falls below the threshold for major depressive disorder and that has lasted without any period of remission for at least 2 years). Here, antidepressants appear to have an advantage over placebo to the same degree as with major depressive disorder (Reference Lima and MoncrieffLima & Moncrieff, 2002). This suggests that the difference between dysthymia and minor depression is the relative lack of the placebo response/natural resolution seen with the former.
Although there is still some debate about the issue, it is possible to argue that the difference between a sustained response to drug and to placebo is quantitative rather than qualitative and that antidepressants increase the probability of recovering from depression. Where the base-rate probability for improvement is high (i.e. acute mild depression) then any added advantage from taking an antidepressant is low and difficult to detect. At higher degrees of severity and chronicity of depression, the probability of placebo response/natural resolution is lower.
Antidepressants are able to trigger recovery, so that the drug–placebo difference is larger, easier to detect and more clearly of clinical relevance. The fact that greater duration and severity of depression result in a greater drug–placebo difference does not, of course, imply that these patients do better when treated than those with milder depression, because both factors are also predictors of overall poorer outcome (Reference Anderson, Nutt and DeakinAnderson et al, 2000). What is emphasised is the need to treat these patients vigorously to improve outcome.
‘Zones of uncertainty’ in benefits from antidepressants
It is unlikely that there is a discrete threshold above which antidepressants suddenly become beneficial. It is better to consider that for any individual patient there is a ‘zone of uncertainty’ at the milder, non-chronic end of the spectrum. It is important to recognise this because all guidelines have the clear message that major depressive disorder should be treated and that antidepressants are the first-line treatment and need to be continued for 6 months after remission. Adopting this duration of treatment means that starting antidepressants is not a trivial undertaking and premature discontinuation is an important reason for the high rate of ‘inadequate’ treatment described. The danger that many people may be given drugs unnecessarily must be balanced against the benefit others will receive. Acknowledging the zone of uncertainty opens the way for discussing with the patient what has been called a period of ‘watchful waiting’, with treatment if the depression does not improve or consideration of simple alternative non-drug treatment, such as problem-solving, which has been shown to be effective in mild-to-moderate major depressive disorder (Reference Anderson, Nutt and DeakinAnderson et al, 2000).
However, even the zone of uncertainty is difficult to define in clinical practice. Decisions about severity, or even about minor depression v. major depressive disorder, depend very much on the experience and expertise of the clinician. For example, the severity of depression seen in primary care is lower than that seen in secondary care and it is likely that, on average, general practitioners (GPs) will judge people as having more severe depression than will psychiatrists.
The typical severity of depression seen in primary care appears to fall in the middle of the zone of uncertainty, making it difficult, if not impossible, to be sure whether a large proportion of patients will truly benefit from antidepressants. GPs appear to be better at detecting more severe depression than is often suggested and the question should at least be raised as to whether the thrust of educative efforts would be better aimed at treating those who have been detected, or are not improving, rather than widening the net to include milder cases.
The issue of duration of depression is also fraught with difficulty and the diagnosis of dysthymia is not made readily by most psychiatrists in the UK, let alone GPs. It is a difficult diagnosis on a practical level and it can be hard to exclude an episode of major depressive disorder or remission at some stage during the 2 years of mood disturbance required to make the diagnosis (both events exclude dysthymia). How long minor depression needs to persist before there is an advantage to treating with antidepressants is currently unknown and is a second zone of uncertainty. Open-minded clinical judgement is therefore needed in making decisions about initiation of antidepressants in many, possibly most, cases of depression in primary care, with current evidence only helping towards the extremes of severity and duration.
Treating non-response
Non-response or inadequate response to antidepressants probably occurs in 50–70% of patients with moderate-to-severe depression (depending on the timescale of assessment) and yet we have very little evidence to guide us in what to do. A major problem is a lack of consensus on the definition of non-responsiveness or treatment resistance, but most schemes have four stages of classification for increasing treatment resistance (Box 2).
Studies in this area are plagued by two main deficits. The first is small size and the second is variations in duration of prior treatment and definition of treatment resistance. There is little information on the difficult issue of how long to persist in the face of inadequate response. Many, particularly older, studies often required only 3 or 4 weeks but it is easy to see that, within this short time-period, some patients may be on a trajectory to improvement but have not yet reached a definition of ‘response’.
Box 2 Classification of treatment for non-response/resistance
Different detailed schemes are used but in general they include four stages:
-
• inadequate treatment (i.e. not resistance to treatment)
-
• non-response to an adequate trial of a single agent
-
• degrees of treatment-resistant depression (non-response to adequate trials of two or more agents from different classes, augmentation, electroconvulsive therapy (ECT))
-
• chronic refractory depression (non-response to multiple treatments, including augmentation and ECT with the depression lasting for more than 2 years)
No improvement at 4 weeks and only partial, insufficient response at 6–8 weeks is often suggested as a guide to halt the first treatment (Reference Anderson, Nutt and DeakinAnderson et al, 2000) but a significant proportion of people continue to respond over the next 6 weeks on an unchaged drug and dose (Reference Ferreri, Lavergne and BerlinFerreri et al, 2001; Reference Licht and QvitzauLicht & Qvitzau, 2002). It appears that the cumulative probability of adequate response levels off with length of treatment and the challenge is to choose the optimal time when altering treatment offers a better chance of response.
Therefore, the four main options for non- or inadequate response are: no change; dose increase; switching drug treatment; and combining drug treatments (Box 3). The term ‘augmentation’ tends to be used to describe combinations where the added drug is believed to have low efficacy on its own but in some way boosts the activity of the antidepressant. This may not always be a sustainable distinction, as in the case of lithium. Empirical evidence about the efficacy of each strategy is pitifully slim and is slimmer still for comparisons between strategies.
Box 3 Utility of strategies for treatment non-response
Continuing the same antidepressant at the same dose
Probability of response decreases after 4–6 weeks and appears minimal after 12 weeks.
Increasing the dose of antidepressant
There is a lack of evidence of efficacy but it is reasonable to exclude an inadequate dose for an individual and to ‘buy time’ to ensure that the trial is sufficient.
Switching antidepressant
Controlled evidence is conflicting, of poor quality and less positive than the 50% often quoted for open studies. A placebo effect or observer bias after switching may be common.
A switch from a monoamine reuptake inhibitor to a monoamine oxidase inhibitor appears most effective but studies are very small.
Augmenting/combining antidepressants
There are many small studies and the quality of evidence is not good. Augmentation with lithium, and possibly tryptophan, and some combinations of antidepressants seem the most promising. Buspirone, pindolol and thyroid hormones are of questionable efficacy. Positive results with hormonal/omega-3 fatty acid augmentation need to be replicated.
Increasing the dose
A decision on whether or not to increase the dose of antidepressant is likely to be highly dependent on the length of treatment and the drug involved. The practice of increasing the dose of tricyclic antidepressants (TCAs) and venlafaxine comes largely from evidence which suggests greater efficacy at higher doses in some circumstances (Reference Mendels, Johnston and MattesMendels et al, 1993; Reference Anderson, Nutt and DeakinAnderson et al, 2000) but this has not been tested directly. In general, the SSRIs, with the possible exception that citalopram and paroxetine, are considered to have a flat dose–response within the usual therapeutic dose range (Reference Tignol, Stoker and DunbarTignol et al, 1992; Reference MontgomeryMontgomery, 1995). The few published RCTs are not helpful (Table 1) and an increase in the dose probably has to be a pragmatic decision to exclude inadequate dose as a reason for non-response, while taking into account side-effects and the need to keep the patient engaged in treatment.
When interpreting open studies, there is clearly a ‘response’ in prior non-responders as a result of the intervention itself, with considerable improvement seen in patients who are randomised to continue blind treatment with the same drug at the same dose (Reference Dornseif, Dunlop and PotvinDornseif et al, 1989; Reference Licht and QvitzauLicht & Qvitzau, 2002). It is not clear whether this is a ‘placebo’ response on the part of the patient, observer bias, or a combination of the two.
Switching antidepressant
In considering the evidence for switching antidepressants, open studies tend to describe response rates of about 50% after a change is made. Controlled evidence is confounded by such issues as length of previous treatment and definition of treatment resistance, together with study design and the number of potential switches that could be made.
Most blinded studies have used a cross-over design or randomisation to two new antidepressants in patients failing to respond in trials of other antidepressants. These studies are therefore not controlled for any response that might have occurred if treatment with the original antidepressant had been continued. An ideal design is to compare patients randomised to continue the previous antidepressant or to switch to an alternative drug. However, to my knowledge, this has only been done is a single study (Reference Ferreri, Lavergne and BerlinFerreri et al, 2001). Following non-response to 6 weeks of fluoxetine, switching to mianserin resulted in a 48% response rate compared with 37% in those continuing on fluoxetine for a further 6 weeks. Even in this case, however, the long half-life of fluoxetine means that the first few weeks after the switch could be considered to be combination treatment. Nevertheless, it is worth keeping the latter figure in mind when interpreting studies of switching antidepressants.
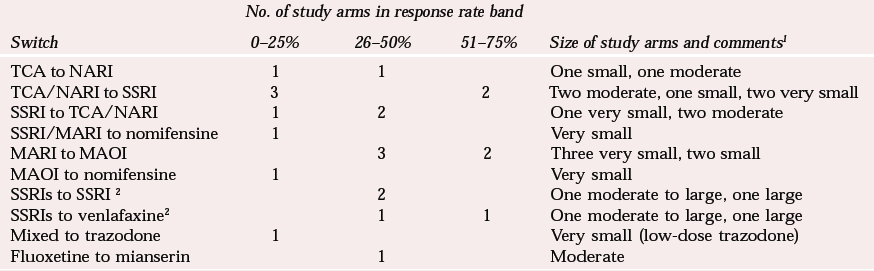
Table 2 illustrates the difficulties in making sense of the randomised studies that are available. In an attempt to compare studies, I have roughly divided the response rates after switching into 25% bands. This does not reveal any very consistent pattern, although the best results (in small studies) are for a switch from monoamine reuptake inhibitors to monoamine oxidase inhibitors. The strategy of switching from an SSRI to a TCA receives only modest support and that for switching from a TCA to an SSRI gives conflicting results. One study of venlafaxine against paroxetine in treatment-resistant patients (Reference Poirier and BoyerPoirier & Boyer, 1999) suggested that it might be better to switch to venlafaxine than to an SSRI, but a more recent study, presented only in abstract form, found no overall benefit for venlafaxine over citalopram in SSRI non-responders, with only a modest response to both drugs over 12 weeks (Reference Lenox-Smith, Schaeffer and ReynoldsLenox-Smith et al, 2001). A subgroup analysis of more severely ill patients did, however, favour venlafaxine.
It might be expected that the response to switching would be poorer with greater prior treatment resistance and there is a suggestion of this in the results. However, different study designs and lack of clarity in definitions of treatment resistance make conclusions difficult.
Augmenting or combining antidepressants
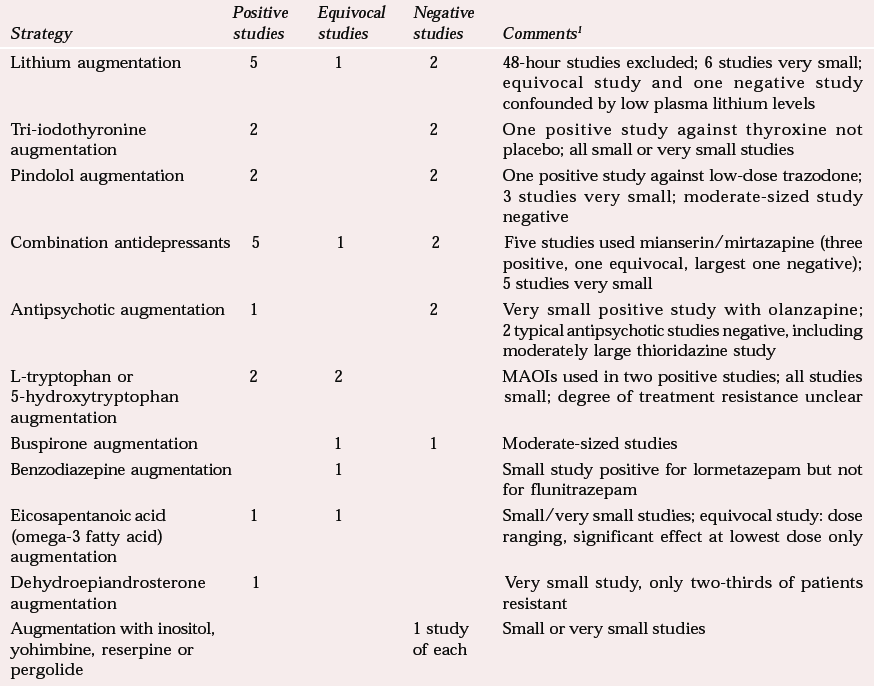
A number of meta-analyses and reviews of augmentation in treatment-resistant patients give positive results for lithium (Reference Austin, Souza and GoodwinAustin et al, 1991; Reference Bauer and DopfmerBauer & Dopfmer, 1999) and more equivocal results for tri-iodothyronine (Reference Aronson, Offman and JoffeAronson et al, 1996) and pindolol (Reference McAskill, Mir and TaylorMcAskill et al, 1998). Table 3 illustrates the diversity of strategies attempted, from which it is difficult to draw general conclusions.
Combination of antidepressants, particularly monoamine reuptake inhibitors, with the 5-hydroxytryptamine2 (5-HT2) and α2 receptor blocking antidepressants mirtazapine or mianserin looks promising, perhaps because of the combination of different action mechanisms, although there are possible symptomatic effects on sleep and appetite. The 5-HT precursors tryptophan and 5-hydroxytryptophan should not be discounted, particularly in combination with monoamine oxidase inhibitors (MAOIs). Other positive results await replication.
Which strategy?
Unfortunately, we have little guidance as to which is the most promising strategy. In practice, dose increase is probably reasonable as a first step, to ensure that an adequate dose is given for that patient for an adequate period. The choice is then between switching or augmenting/combining antidepressants. Most guidelines and algorithms suggest switching before augmenting. Both have advantages and disadvantages, quite apart from the evidence of efficacy. Switching avoids potential toxicity or interactions that could occur with combinations but it requires care in the changeover of drugs, which can cause delay and discontinuation reactions; these are avoided with the addition of a second drug. In cases of partial response, it may seem better to augment than to start afresh, whereas tolerability problems with the first drug favours switching.
It is therefore a matter of judgement as to which strategy should be used until there is evidence to choose between them. Of interest, there is an effectiveness trial under way comparing ‘next-step’ treatments, STAR∗D or Sequenced Treatment Alternatives to Relieve Depression (http://www.edc.gsph.pitt.edu/stard). A small, open, prospective naturalistic trial in the USA found a non-significant but potentially clinically useful advantage to combination treatment (56% response) compared with switching (45% response) (Reference Posternak and ZimmermanPosternak & Zimmerman, 2001). If we return to the clinical reality of patients with higher degrees of treatment resistance, the evidence we have is of limited assistance and it is important to consider therapeutic trials in the light of individual need while walking the line between over-optimism and therapeutic nihilism.
What we need to acknowledge is that common strategies of giving very high doses with sequences of newer antidepressants, then TCAs, then MAOIs (in various combinations with augmentation) are more art than science, but art that needs to keep on searching for scientific underpinning.
How long should antidepressants be continued?
Numerous controlled trials have established the need to continue antidepressants after remission of depression in order to prevent relapse and this is accepted wisdom. Indeed, in Europe, an antidepressant cannot be licensed unless it has been shown to be efficacious in continuation treatment. Nevertheless, we are left with the problem of how to identify those who will benefit significantly from continuation. In other words, if someone has not benefited acutely from the drug itself (i.e. had a spontaneous improvement or a placebo response), does continuation treatment prevent relapse?
Reference Quitkin, Stewart and McGrathQuitkin et al(1993) found that relapse during the continuation phase was three times higher in patients responding to, and continued on, placebo than in those responding to, and continued on, antidepressants; this rate is similar to that seen when responders to an antidepressant are switched to placebo (Reference Montgomery, Rasmussen and TanghojMontgomery et al, 1993). However, Reference Stewart, Quitkin and McGrathStewart et al(1998) found that patients treated with fluoxetine who showed an early, abrupt, inconsistent response when treated with the drug (which has been attributed to placebo effects) did no better on continued fluoxetine treatment than on continuation placebo. In contrast, those showing a progressive sustained response with fluoxetine treatment (associated with the drug or a spontaneous response) did show a benefit from continuing fluoxetine compared with placebo and fared better than patients with early, abrupt, inconsistent response.
These studies raise the possibility that for patients with at least moderately severe depression, those who improve gradually and in a sustained fashion without antidepressant treatment might have a reduced rate of relapse if they are started on an antidepressant. However, to my knowledge, this has not been tested directly, not even in the obvious study where those responding to placebo are randomised to active or placebo treatment.
When we consider patients with milder depressions and those with fluctuating courses and inconsistent responses, there is a lack of evidence for continuation of antidepressants. The fact that the majority of patients treated for depression in primary care stop antidepressants within a few weeks may not be the real issue. Perhaps we should be more concerned with identifying the patients who need to remain on antidepressants rather than trying to persuade everyone to stay on months of treatment.
Assessing potential clinical benefit from continuation/maintenance antidepressants
It is standard teaching to distinguish between relapse and recurrence of depression; the former is a return of the original episode, the latter is the occurrence of a new episode. Besides frequently being difficult to make in practice, this distinction may not be very helpful in considering continuing drug treatment. There is a tendency to dogmatically recommend that antidepressants be continued for 6 months, whatever the circumstances, and to treat the question of longer-term maintenance to prevent recurrence as a separate (and frequently neglected) issue.
Preliminary results from a Cochrane review comparing continuation of antidepressants with their discontinuation (Reference Carney, Geddes and DaviesCarney et al, 2001) suggest that the degree of benefit (relative risk reduction) from continuing antidepressants remains about the same over time (at least up to the 3 years of the longest study). The absolute benefit depends on the risk of relapse/recurrence. If this is high, as it is on average in the 3–4 months after initial remission from a depressive episode, then the benefit will be considerable. If the risk is lower, then any benefit will be less apparent, difficult to detect statistically and its clinical relevance will not be clear.
The difficulty is determining the risk of relapse for an individual. What is certain, however, is that more factors are important than simply time from the last episode or the total number of episodes, particularly severity of initial illness, duration, persisting symptoms, and social and personality factors (Reference Anderson, Nutt and DeakinAnderson et al, 2000). The consequences of a relapse/recurrence, such as disruption to the family, work or study, also need to be considered when determining the value of preventing the return of depression. Therefore, an assessment needs to be made when advising a patient about the duration of antidepressant treatment, first of the risk of relapse over time and second of the importance of its prevention.
Continuing with antidepressants probably cuts the risk of relapse to about 40% of what it would be without treatment. This then allows an informed discussion with the patient about the pros and cons of continuing antidepressants. Someone with a moderately severe, short-lived depression strongly related to a life event that has resolved, who recovers completely and is financially independent, has a different balance of risks and benefits when considering whether to stop antidepressants than someone who has had a severe episode, some persisting symptoms, continuing social difficulties and is the sole wage-earner in the family. The risk of recurrence and resulting adverse consequences in the first case are likely to be considerably lower than in the second and the benefit from continuing antidepressants would be considered more marginal.
Box 4 Key areas of clinical uncertainty in the treatment of depression with antidepressants
What is the threshold of severity of major depression above which antidepressants are of practical benefit?
How long does milder depression need to last before treatment with antidepressants becomes beneficial?
What is the optimal drug treatment strategy to adopt when a patient has failed to respond to an antidepressant?
For how long should antidepressant use be continued?
Conclusions
I have tried to outline some important areas that I believe remain problematic in our use of antidepressants in the treatment of depressive disorders (Box 4). Although a few are matters of further evidence (e.g. which combination treatments are effective), most will be only partly illuminated by research and are always likely to remain a matter of clinical judgement (which should, nevertheless, be as informed as possible).
Table 1 Randomised controlled trials of increasing antidepressant dose in non-responders to treatment

| Antidepressant | Positive studies | Equivocal studies | Negative studies | Comments 1 |
|---|---|---|---|---|
| Selective serotonin reuptake inhibitors | 1 (fluoxetine) | 1 (paroxetine) | 2 (fluoxetine, sertaline) | Positive study very small, others moderate to large. In equivocal study and one of the negative studies dose was increased after only 3 weeks |
| Maprotiline | 1 | Moderate size study, dose was increased after only 3 weeks |
Table 2 Controlled studies involving switching antidepressants in treatment of non-responders
| No. of study arms in response rate band | ||||
|---|---|---|---|---|
| Switch | 0–25% | 26–50% | 51–75% | Size of study arms and comments 1 |
| TCA to NARI | 1 | 1 | One small, one moderate | |
| TCA/NARI to SSRI | 3 | 2 | Two moderate, one small, two very small | |
| SSRI to TCA/NARI | 1 | 2 | One very small, two moderate | |
| SSRI/MARI to nomifensine | 1 | Very small | ||
| MARI to MAOI | 3 | 2 | Three very small, two small | |
| MAOI to nomifensine | 1 | Very small | ||
| SSRIs to SSRI 2 | 2 | One moderate to large, one large | ||
| SSRIs to venlafaxine2 | 1 | 1 | One moderate to large, one large | |
| Mixed to trazodone | 1 | Very small (low-dose trazodone) | ||
| Fluoxetine to mianserin | 1 | Moderate | ||
Table 3 Augmentation or combination antidepressant placebo-controlled randomised controlled trial in non-responders to antidepressants
| Strategy | Positive studies | Equivocal studies | Negative studies | Comments 1 |
|---|---|---|---|---|
| Lithium augmentation | 5 | 1 | 2 | 48-hour studies excluded; 6 studies very small; equivocal study and one negative study confounded by low plasma lithium levels |
| Tri-iodothyronine augmentation | 2 | 2 | One positive study against thyroxine not placebo; all small or very small studies | |
| Pindolol augmentation | 2 | 2 | One positive study against low-dose trazodone; 3 studies very small; moderate-sized study negative | |
| Combination antidepressants | 5 | 1 | 2 | Five studies used mianserin/mirtazapine (three positive, one equivocal, largest one negative); 5 studies very small |
| Antipsychotic augmentation | 1 | 2 | Very small positive study with olanzapine; 2 typical antipsychotic studies negative, including moderately large thioridazine study | |
| L-tryptophan or 5-hydroxytryptophan augmentation | 2 | 2 | MAOIs used in two positive studies; all studies small; degree of treatment resistance unclear | |
| Buspirone augmentation | 1 | 1 | Moderate-sized studies | |
| Benzodiazepine augmentation | 1 | Small study positive for lormetazepam but not for flunitrazepam | ||
| Eicosapentanoic acid (omega-3 fatty acid) augmentation | 1 | 1 | Small/very small studies; equivocal study: dose ranging, significant effect at lowest dose only | |
| Dehydroepiandrosterone augmentation | 1 | Very small study, only two-thirds of patients resistant | ||
| Augmentation with inositol, yohimbine, reserpine or pergolide | 1 study of each | Small or very small studies |
Multiple choice questions
-
1 Relating to the quality of evidence available from treatment trials:
-
a RCTs are designed to minimise the effects of bias on outcomes
-
b the results of RCTs can be accepted at face value
-
c the nature of the outcome measures used in psychiatry means that the results are usually robust
-
d unreplicated small studies should not be trusted
-
e RCTs usually involve typical patients.
-
-
2 Patients are likely to benefit from being treated with an antidepressant rather than placebo in:
-
a severe major depression
-
b minor depression
-
c dysthymia
-
d mild major depression
-
e chronic major depression
-
-
3 Reasonable strategies in patients not responding to 6 weeks’ treatment with an antidepressant include:
-
a continuing the same treatment
-
b increasing the dose
-
c switching antidepressant
-
d augmenting the antidepressant with another drug
-
e combining antidepressants.
-
-
4 In studies of antidepressant augmentation/combination for treatment-resistant depression:
-
a lithium augmentation has the strongest evidence base
-
b pindolol augmentation is clearly effective
-
c combination of a monoamine reuptake inhibitor with mianserin/mirtazapine may be useful
-
d tri-iodothyronine augmentation is clearly ineffective
-
e studies of augmentation with tryptophan are uniformly negative.
-
-
5 In continuation/maintenance treatment with antidepressants:
-
a all patients should continue for 6 months on an antidepressant after remission following acute treatment
-
b patients with more than two episodes of major depression should routinely have maintenance antidepressants for at least 5 years
-
c continuing antidepressant treatment appears to reduce the risk of relapse and recurrence by a similar proportion, whatever the underlying risk
-
d the underlying risk of relapse should not be a major factor in the decision to continue antidepressant treatment
-
e advice about continuing antidepressants should be individually tailored to each patient's circumstances as far as possible.
-
MCQ answers

| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | T | a | T | a | T | a | T | a | F |
| b | F | b | F | b | T | b | F | b | F |
| c | F | c | T | c | T | c | T | c | T |
| d | T | d | F | d | T | d | F | d | F |
| e | F | e | T | e | T | e | F | e | T |







eLetters
No eLetters have been published for this article.