The normal sexual response is conventionally divided into the four phases listed below, and disorders can occur at one or more of these phases.
-
(1) Desire: typically this consists of fantasies about, and the desire to have, sexual activity.
-
(2) Excitement: a subjective sense of sexual pleasure and accompanying physiological changes, namely penile tumescence and erection in men and pelvic vasocongestion, swelling of the external genitalia, vaginal lubrication and expansion in women.
-
(3) Orgasm: this is when sexual pleasure peaks, with release of sexual tension and rhythmic contraction of the perineal muscles and reproductive organs. In men, the sensation of ejaculatory inevitability is followed by ejaculation of semen. In women, contractions of the outer third of the vaginal wall occur.
-
(4) Resolution: a sense of muscular relaxation and general well-being. Men are physiologically refractory to erection and orgasm for a variable period after orgasm, whereas women may respond to further stimulation.
The ICD–10 (World Health Organization, 1992) uses the term ‘sexual dysfunction’ to cover the ways in which an individual is unable to participate in a sexual relationship as he or she would wish. This classification has 10 subdivisions (F52.0–F52.9), each describing different forms of dysfunction. The DSM–IV (American Psychiatric Association, 1994) uses a similar scheme. Whenever possible, doctors should specify which form of sexual dysfunction is present, as these have differing causes and require different treatment approaches.
Some types of dysfunction occur in both men and women, although women tend to present with complaints about the subjective quality of sexual experience (e.g. lack of desire), whereas men often describe the failure of a specific response (such as erection) but a continuing sexual desire.
Epidemiology of sexual dysfunction
This area has not been studied extensively. Reference NathanNathan (1986) evaluated 22 studies of sexual behaviour in the general population but concluded that methodological problems in the surveys meant that only broad estimates could be made. The prevalence of inhibited sexual desire was 16% for men, 35% for women; for erectile dysfunction and premature ejaculation the prevalence values were 10–20% and 35% of men, respectively; female orgasmic difficulties had a prevalence of 5–15%.
A US study found that sexual dysfunction in the general population is more prevalent in women (43%) than men (31%) (Reference Laumann, Park and RosenLaumann et al, 1999). Using latent class analysis, symptoms during the previous 12 months could be grouped into three categories. In women, these were low sexual desire (22% prevalence), arousal or excitement problems (14%) and sexual pain (7%); in men, they were premature ejaculation (21%), erectile dysfunction (5%) and low sexual desire (5%).
Reported rates of sexual dysfunction vary considerably, reflecting differences in the study population and types of dysfunction being assessed. Other studies with data for both genders show a higher prevalence of sexual dysfunction in women than in men (Reference Ernst, Foldenyi and AngstErnst et al, 1993; Reference Dunn, Croft and HackettDunn et al, 1998) and confirm the results for the most common sexual dysfunction in both men and women.
Many factors influence the reported incidence of sexual dysfunction. The first is the method of enquiry. In a prospective study of out-patients with depression, for example, the incidence of sexual dysfunction was 14% when relying on spontaneous reporting. However, this rose to 58% when patients were questioned directly by doctors (Reference Montejo-González, Liorca and IzquierdoMontejo-González et al, 1997). The second factor is that the expectation people have of their sexual performance and their willingness to discuss problems vary between cultures (Reference Bhugra and De SilvaBhugra & De Silva, 1993). In the third place, many terms used to define sexual dysfunction are subjective and partly dependent on ideas of what is ‘normal’. Finally, temporal trends can occur as increased awareness of sexual matters and availability of treatment increase the number of those who perceive themselves to be suffering from sexual dysfunction.
Sexual dysfunction in depression
Depression is characterised by loss of interest, reduction in energy, lowered self-esteem and inability to experience pleasure: irritability and social withdrawal may impair the ability to form and maintain intimate relationships. It would be surprising if this constellation of symptoms did not produce difficulties in sexual relationships (Reference Baldwin, Thomas and BirtwistleBaldwin et al, 1997).
In an early study of 132 patients with depressive disorders, loss of sexual interest (characterised by loss of libido or decrease of sexual desire or potency) was reported by 72% of patients with unipolar disorder and 77% of those with bipolar disorder (Reference Casper, Redmond and KatzCasper et al, 1985). Loss of sexual desire may be the presenting complaint in some patients who are found to have significant depressive symptoms only after direct questioning. In others, low sexual desire may pre-date other features of depression (Reference Schreiner-Engel and SchiaviSchreiner-Engel & Schiavi, 1986).
Comparative studies indicate higher levels of sexual dysfunction in patients with depression than in controls (Table 1). Although the incidence of specific types of sexual dysfunction varies across studies, loss of sexual desire may be more common than disorders of arousal and orgasm. For example, in one comparative study, changes in libido were significantly more common in patients with depression, but the prevalence of impotence, orgasmic or ejaculatory problems did not differ from controls (Reference Mathew and WeinmanMathew & Weinman, 1982). The prospective Zurich cohort study (Reference Ernst, Foldenyi and AngstErnst et al, 1993) showed that the overall prevalence of sexual problems in subjects with depression (including major depression, dysthymia and recurrent brief depression) was about twice that in controls (50% v. 24%). This difference encompassed emotional problems, sexual dysfunction and both decreased and increased libido. The study findings were from a group of young people (28–35 years old) and are not necessarily applicable to older age groups (Reference AngstAngst, 1998).
Table 1 Comparative studies of sexual dysfunction in patients with depression and in controls (terminology is taken from the sources)
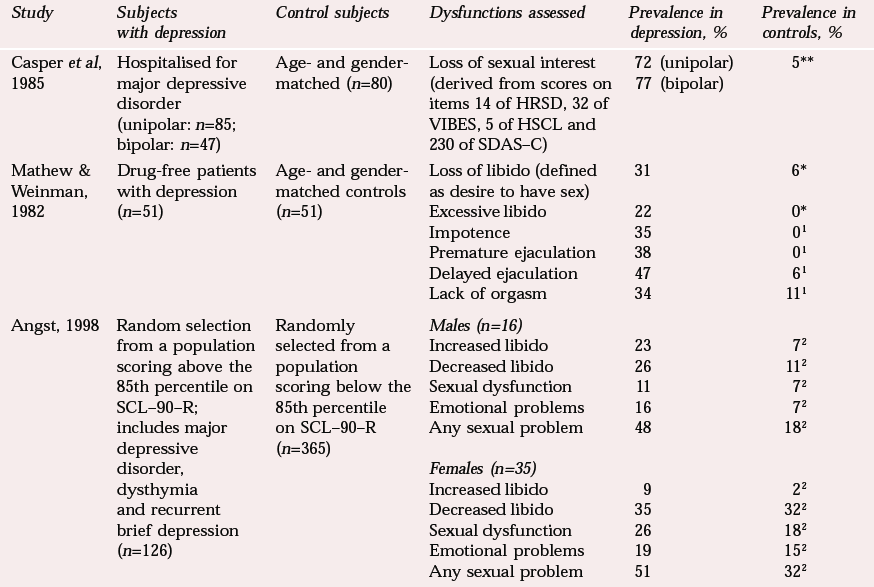
| Study | Subjects with depression | Control subjects | Dysfunctions assessed | Prevalence in depression, % | Prevalence in controls, % |
|---|---|---|---|---|---|
| Reference Casper, Redmond and KatzCasper et al, 1985 | Hospitalised for major depressive disorder (unipolar: n=85; bipolar: n=47) | Age- and gender- matched (n=80) | Loss of sexual interest (derived from scores on items 14 of HRSD, 32 of VIBES, 5 of HSCL and 230 of SDAS–C) | 72 (unipolar) 77 (bipolar) |
5∗∗ |
| Reference Mathew and WeinmanMathew & Weinman, 1982 | Drug-free patients with depression (n=51) | Age- and gender- matched controls (n=51) | Loss of libido (defined as desire to have sex) | 31 | 6∗ |
| Excessive libido | 22 | 0∗ | |||
| Impotence | 35 | 01 | |||
| Premature ejaculation | 38 | 01 | |||
| Delayed ejaculation | 47 | 61 | |||
| Lack of orgasm | 34 | 111 | |||
| Reference AngstAngst, 1998 | Random selection from a population scoring above the 85th percentile on SCL–90–R; includes major depressive disorder, dysthymia and recurrent brief depression (n=126) | Randomly selected from a population scoring below the 85th percentile on SCL–90–R (n=365) |
Males (n=16)
Increased libido Decreased libido Sexual dysfunction Emotional problems Any sexual problem Females (n=35) Increased libido Decreased libido Sexual dysfunction Emotional problems Any sexual problem |
23 26 11 16 48 9 35 26 19 51 |
72
112 72 72 182 22322182152322 |
The Zurich study also compares the prevalence of sexual problems in untreated patients and patients receiving either medication (50% benzodiazepines, 50% antidepressants) or psychotherapy. Sexual problems were more prevalent in the 78 patients who received treatment (62%) than in the 122 who did not (45%) and both groups had a higher prevalence of sexual dysfunction than did the 326 controls (26%). No statistically significant differences were found in the prevalence of any form of sexual dysfunction between patients treated with medication or psychotherapy alone (Reference AngstAngst, 1998).
Sexual function in schizophrenia
There have been few studies of sexual function and satisfaction in people with schizophrenia, and comparisons of untreated and treated patients are rare (Reference Baldwin and BirtwistleBaldwin & Birtwistle, 1997). In a comparison of successive cohorts of patients with schizophrenia, rates of marriage and fertility increased in men and women across all age groups; the authors attributed this to reduced time spent in hospital and more-flexible social attitudes (Reference Erlenmayer-Kimmling, Nicol and RainerErlenmayer-Kimmling et al, 1969). However, clinicians traditionally believed that patients who engaged in sexual relations were in danger of exacerbating their illness (Reference Pinderhughes, Grace and ReynaPinderhughes et al, 1972), and in many institutions sexual activity was discouraged, partly owing to fear of exploitation and pregnancy in vulnerable patients (Reference Akhtar, Crocker and DickeyAkhtar et al, 1977).
Although sexual delusions and somatic hallucinations can form part of the psychopathology of schizophrenia, the onset of psychosis is often associated with reduced sexual activity (Reference Rozan, Tuchin and KurlandRozan et al, 1971). Persistent psychosis is also associated with reductions in sexual interest, activity and satisfaction (Reference Lyketsos, Sakka and MailisLyketsos et al, 1983). Treated and untreated male patients reported decreased sexual desire and behaviour and increased rates of premature ejaculation than did controls. In a comparison with untreated male patients, depot antipsychotic treatment increased sexual thoughts and desire but also resulted in sexual dysfunction (Reference Aizenberg, Zemishlany and DorfmanAizenberg et al, 1995). In another comparison, sexual dysfunction was more common in female patients than in controls, but not in those patients with a satisfactory overall relationship with a sexual partner (Reference RabochRaboch, 1986).
It is unclear whether sexual dysfunction is more or less common in patients with schizophrenia than in those with other forms of mental disorder. A small survey (n=56) of in-patients found that 62% of men and 25% of women with schizophrenia reported sexual or relationship problems, compared with 63–75% of men and 50–100% of women with affective disorder (Reference Bhui, Puffet and HerriotBhui et al, 1995). In a larger study (n=173), sexual problems were significantly more common in patients with schizophrenia who were receiving treatment than in patients with anxiety disorders who were not receiving treatment, but they were as common as in patients with opiate dependence who were receiving methadone (Reference Teusch, Scherbaum and BohmeTeusch et al, 1995).
Neurotransmitters and sexual function
Many neurotransmitters and hormones are thought to play a role in maintaining normal sexual function, although their precise actions are not fully understood. They include dopamine, noradrenaline, serotonin (5-hydroxytryptamine; 5-HT), acetylcholine, γ-aminobutyric acid, oxytocin, nitric oxide, arg-vasopressin, angiotensin II, gonadotrophin-releasing hormone, substance P, neuropeptide Y and cholecystokinin. Of these, dopamine, serotonin and nitric oxide may have the most important roles in the pathophysiology and treatment of sexual dysfunction arising from antidepressant and antipsychotic drugs (Reference Baldwin, Thomas and BirtwistleBaldwin et al, 1997).
Increased levels of central dopamine can increase sexual arousal and enhance penile erection. Centrally acting dopaminergic compounds have been shown to facilitate sexual behaviour in animal models and the dopaminergic agonist bromocriptine can reduce sexual dysfunction in men with hyperprolactinaemia associated with chronic renal failure (Reference Ramirez, Butcher and NewtonRamirez et al, 1985). Dopamine antagonists, such as most antipsychotics, can reduce sexual performance both directly and indirectly through inducing hyperprolactinaemia (Reference SegravesSegraves, 1989). Increased central serotonergic neurotransmission can reduce sexual behaviour. Activation of 5-HT2 receptors mediates inhibitory actions on sexual behaviour in animal models and 5-HT2 antagonists, such as cyproheptadine, and 5-HT1a agonists, such as buspirone, can reverse sexual dysfunction induced by selective serotonin reuptake inhibitors (SSRIs).
Penile erection involves vasodilatation arising from relaxation of smooth muscle in the corpus cavernosum. The key mediator for this is nitric oxide, which acts by increasing the cellular level of the cyclic nucleotide guanosine monophosphate (cGMP). Increases in cGMP levels can be also be effected by inhibition of phosphodiesterases, which are the enzymes involved in cyclic nucleotide breakdown. Sildenafil is a phosphodiesterase-5 inhibitor that enhances nitric-oxide-mediated vasodilatation in the corpus cavernosum by inhibiting breakdown of cGMP.
Sexual side-effects of conventional antipsychotics
Treatment-emergent sexual problems have been described as ‘the unspoken side-effect of antipsychotics’ (Reference Peuskens, Sienaert and De HeftPeuskens et al, 1998). A questionnaire survey of patient satisfaction with antipsychotic drug treatment found that 43% of 202 respondents reported sexual dysfunction (Reference WallaceWallace, 2001). Another survey suggested that doctors underestimate the sexual and menstrual adverse effects of antipsychotic drugs (Reference HellewellHellewell, 1998).
It is unclear whether the untoward sexual effects of antipsychotic drugs arise mainly from direct pharmacological effects (such as dopamine receptor antagonism) or from secondary endocrine disturbances. Hyperprolactinaemia associated with prolactinomata can result in loss of sexual desire, erectile failure and reduced spermatogenesis in men; in women it can lead to altered ovarian cyclic function, amenorrhoea, reduced sexual desire and hirsutism (Reference PettyPetty, 1999). The claim that antipsychotic-induced hyperprolactinaemia may increase the risk of osteoporosis is not yet substantiated, as little research has been performed (Reference Dickson and GlazerDickson & Glazer, 1999). Conventional antipsychotic drugs can increase prolactin levels to a range associated with sexual dysfunction in non-psychiatric patients, so hyperprolactinaemia is a probable explanation of some of the sexual dysfunction seen during treatment with antipsychotics. However, in many patients, the increase in prolactin levels with antipsychotic treatment is only transient. An early small (n=27) study of male patients with schizophrenia found that those with erectile failure had higher prolactin levels than those without sexual problems (Reference Arato, Erdos and PolgarArato et al, 1979). In a subsequent investigation in male and female patients, elevated prolactin levels were associated with sexual dysfunction in men and menstrual disturbances in women (Reference Ghadirian, Chouinard and AnnableGhadirian et al, 1982). A study of sexual function in patients taking conventional neuroleptics found that hyperprolactinaemia was the main cause of sexual dysfunction in women and in men, and that dysfunction occurred in the presence of both normal and elevated prolactin levels. However, elevated prolactin levels were likely to override any other possible causes of sexual dysfunction in these individuals (Reference Smith, O'Keane and MurraySmith et al, 2002).
Treatment with butyrophenones or phenothiazines can result in loss of sexual interest. This probably results from direct effects such as dopamine receptor antagonism and secondary hyperprolactinaemia. Other adverse effects such as sedation, extra-pyramidal effects and weight gain can also reduce sexual desire (Reference Baldwin and BirtwistleBaldwin & Birtwistle, 1997).
Erectile failure can often result from phenothiazine treatment: in one study, 38% of men had difficulty in achieving, and 42% in maintaining, erection (Reference Ghadirian, Chouinard and AnnableGhadirian et al, 1982). Drug-induced priapism results from α-adrenergic blockade combined with anticholinergic activity. Antipsychotic drugs with this profile (e.g. chlorpromazine or thioridazine) can prevent detumescence but can also cause painful prolonged erection of the penis or clitoris. About 20% of cases of drug-induced priapism are due to antipsychotic drugs; the risk seems independent of dosage or duration of treatment (Reference Patel, Mukherji and LeePatel et al, 1996).
Ejaculatory problems are a common side-effect of conventional antipsychotics. Total inhibition of ejaculation is most common (Reference Mitchell and PopkinMitchell & Popkin, 1983) but reduced ejaculatory volume (Reference Ghadirian, Chouinard and AnnableGhadirian et al, 1982) and ‘retrograde’ ejaculation are not unusual. Spontaneous ejaculation in the absence of sexual stimulation can occur with antipsychotics but this is rare (Reference Keitner and SelubKeitner & Selub, 1993). Antipsychotic drug treatment is often associated with qualitative changes in orgasm. For example, painful orgasm has been reported during treatment with thioridazine, trifluoperazine and haloperidol (Reference Baldwin and BirtwistleBaldwin & Birtwistle, 1997).
Sexual side-effects of atypical antipsychotics
Some atypical antipsychotic drugs (e.g. quetiapine) have a notable affinity for 5-HT2 receptors as well as for dopamine D2 receptors. As 5-HT2 antagonists can facilitate sexual behaviour (see below), drugs with this effect would be expected to cause fewer sexual side-effects than those without this property. A comparative study of men with schizophrenia treated with either conventional antipsychotics or clozapine found that clozapine treatment was associated with significantly better sexual functioning, as assessed by frequency of orgasm, enjoyment of sex and sexual satisfaction (Reference Aizenberg, Modai and LandaAizenberg et al, 2001). The efficacy of clozapine in relieving positive and negative symptoms as well as other aspects of psychopathology may also have favourable effects on interpersonal relationships and sexual health.
However, there are case reports of retrograde ejaculation and priapism with clozapine, risperidone and olanzapine (Reference Rosen and HannoRosen & Hanno, 1992; Reference Emes and MilsonEmes & Milson, 1994; Reference Deirmenjian, Erhart and WirshingDeirmenjian et al, 1998; Reference Compton, Saldivia and BerryCompton et al, 2000; Reference Songer and BarclaySonger & Barclay, 2001).
It is uncertain whether any particular atypical antipsychotic drug is more likely to cause sexual side-effects than others. A controlled treatment study (n=339) comparing olanzapine with risperidone found that sexual dysfunction was significantly less common with olanzapine (Reference Tran, Hamilton and KuntzTran et al, 1997). Quetiapine treatment is not associated with hyperprolactinaemia, which may be an advantage. A recent retrospective cross-sectional study indicates that sexual dysfunction is less common with quetiapine (18.2%, mean dose 360.5 mg/day) than with haloperidol (38.1%, 10.6 mg/day), olanzapine (35.3%, 13.5 mg/day) or risperidone (43.2%, 5.3 mg/day) (Reference Bobes, Rejas and GarciaBobes et al, 2001). An analysis of treatment studies with amisulpride indicates similar overall rates of all forms of endocrine disorder with amisulpride and risperidone (4% v. 6%); the rate of erectile failure was significantly lower with amisulpride (1% v. 5%) (Reference Coulouvrat and Dondey-NouvelCoulouvrat & Dondey-Nouvel, 1999). Both amisulpride and risperidone can cause substantial rises in prolactin levels.
Sexual side-effects of antidepressants
Treatment-emergent sexual dysfunction can occur with tricyclic antidepressants, SSRIs and monoamine oxidase inhibitors (MAOIs). Some antidepressants (bupropion, mirtazapine, moclobemide, nefazodone and reboxetine) may be associated with a relatively lower incidence of sexual dysfunction, but most data for these newer drugs come from reviews of clinical trial adverse event databases or work with healthy volunteers (Reference BaldwinBaldwin, 2001).
Few data have been published on sexual dysfunction occurring with tricyclics, although anorgasmia was common in a study of clomipramine in patients with obsessive–compulsive disorder (Reference Monteiro, Noshirvani and MarksMonteiro et al, 1987). The majority of studies have assessed sexual dysfunction with SSRIs, but it is difficult to interpret these findings as the study designs vary considerably. For example, fluoxetine had both the highest (75%) and the lowest (8%) reported prevalence of treatment-emergent sexual dysfunction, one figure being derived from specific questioning about abnormal ejaculation (Reference PattersonPatterson, 1993), the other from spontaneous reports of orgasmic problems (Reference Zajecka, Fawcett and SchaffZajecka et al, 1991). Comparative studies of SSRIs have generally found no significant differences between drugs, but one study reported more sexual dysfunction with sertraline than with fluvoxamine (Reference Nemeroff, Ninan and BallengerNemeroff et al, 1995).
Bupropion may be associated with a low incidence of adverse sexual effects, being significantly superior to sertraline in one study (Reference Croft, Settle and HouserCroft et al, 1999) and to SSRIs in another (Reference Modell, Katholi and ModellModell et al, 1997). A comparative study with nefazodone and sertraline found nefazodone (a 5-HT2 antagonist) to be significantly superior to sertraline on some measures of sexual function (Reference Feiger, Kiev and ShrivastavaFeiger et al, 1996). The findings of a randomised controlled trial comparing moclobemide (a reversible inhibitor of monoamine oxidase type A) with the tricyclic doxepin, together with a study in healthy volunteers and post-marketing surveillance, suggest that moclobemide is relatively free of adverse effects on sexual function (Reference BaldwinBaldwin, 2001).
Antidepressant augmentation or switching studies
Several studies have investigated whether SSRI-associated sexual dysfunction can be resolved by adding, or changing to, a different antidepressant. For example, a retrospective review of 16 patients who complained of sexual dysfunction during SSRI treatment found that 11 (69%) rated their sexual function as much or very much improved when buspirone was added (Reference NordenNorden, 1994).
Mirtazapine (which also possesses 5-HT2 antagonist properties) may be useful in some patients who develop sexual dysfunction with SSRIs. In a study of 20 patients with SSRI-associated sexual dysfunction who switched to mirtazapine, sexual function improved in 9 out of 12 patients (75%) who completed 6-weeks’ treatment, although 6 patients developed irritability and 9 reported sedation (Reference Gelenberg, Laukes and McGahueyGelenberg et al, 1998). A second study, of 11 patients who stopped SSRIs because of sexual problems, found that mirtazapine did not result in the re-emergence of sexual dysfunction (Reference Koutouvidis, Pratikakis and FotiadouKoutouvidis et al, 1999). These observations are supported by findings in a group of 25 out-patients with depression, indicating that mirtazapine had beneficial effects on sexual function (Reference Boyarsky, Haque and RouleauBoyarsky et al, 1999).
The largest body of data is for bupropion, where the findings of one augmentation study and two substitution studies suggest that bupropion can ameliorate SSRI-induced sexual dysfunction (Reference BaldwinBaldwin, 2001). However, not all data are consistent. A retrospective review of 27 patients found that sexual dysfunction occurred in 11 patients (41%) when they were receiving combination bupropion–SSRI treatment, which was not significantly different from the rate (52%) when they were taking either agent alone (Reference Bodkin, Lasser and WinesBodkin et al, 1997). A recent augmentation study with bupropion (150 mg/day) indicates that it can be usefully combined with venlafaxine, paroxetine or fluoxetine (Reference Kennedy, McCann and MasellisKennedy et al, 2002).
Treatment of psychotropic-induced sexual dysfunction
Sexual dysfunction should be assessed sensitively and treatment tailored to individual needs and circumstances. Assessment of a patient who complains of sexual difficulties while taking a psychotropic drug involves the detailed evaluation of a range of factors (Box 1). Several strategies may be beneficial in treatment, including:
Box 1 Assessment of sexual dysfunction in a patient taking a psychotropic drug
Clinical featureRelevant factor
Sexual function before mental health problemsLong-standing erectile failure
Severity of primary psychiatric disorderCurrent mild depressive episode
Severity of comorbid psychiatric disorderCurrent alcohol dependence
Presence of comorbid physical illnessHypertension
Prescribed psychotropic medicationFluoxetine
Prescribed medication for physical illnessAtenolol
Over-the-counter or illicit drug useBenzodiazepine misuse
Overall relationship with sexual partnerFrequent arguments and separations
-
• waiting for the development of tolerance
-
• reduction in dosage
-
• delaying the taking of the drug until after sexual activity
-
• ‘drug holidays’
-
• adjuvant treatments
-
• changing to a different psychotropic drug
-
• behavioural strategies to improve sexual technique
-
• individual psychotherapy
-
• couple therapy.
The role of psychological approaches is outside the scope of this article. The choice of a particular strategy is determined, among other factors, by considering the type and severity of the dysfunction, the nature of the psychiatric diagnosis, the current mental state of the patient, the potential risks of stopping treatment, the risk of untoward drug interactions and the presence or absence of a sexual partner.
Drug holidays, involving brief interruptions of treatment, have been advocated as an approach to SSRI-induced sexual dysfunction (Reference RothschildRothschild, 1995). However, this puts the patient at risk of discontinuation symptoms and possible relapse of depression or schizophrenia. Furthermore, a drug holiday is possible only with drugs with a short half-life. Thus, it is not appropropriate with fluoxetine, for example, where sexual side-effects may not resolve until a few weeks after stopping treatment.
Many adjuvant compounds have been advocated for relieving sexual dysfunction associated with antidepressant or antipsychotic drug treatment, including amantadine, buspirone, cyproheptadine, dexamphetamine, Ginkgo biloba, granisetron, mianserin, neostigmine, olanzapine, prostaglandin E (by intracavernosal injection), sildenafil and yohimbine. However, the results of placebo-controlled studies in this area have generally failed to distinguish between active treatments and placebo. It is wise to be cautious when using unfamiliar treatments. A recent small (n=19) placebo-controlled augmentation study with Ginkgo biloba found no difference between treatments in reversing sexual dysfunction associated with antidepressant treatment (Reference Kang, Lee and KimKang et al, 2002). Another placebo-controlled study found no significant advantage for mirtazapine, yohimbine or olanzapine in relieving sexual dysfunction when taking SSRIs (Reference Michelson, Bancroft and TargumMichelson et al, 2000).
It seems likely that sildenafil may come to have a role in relieving sexual dysfunction associated with psychotropic drugs. In a sub-group of 136 patients with depression included within the placebo-controlled clinical trial database, 76% described improvements with sildenafil, compared with 18% of the group who received placebo (Reference PricePrice, 1999). In an open study, sildenafil was effective in 10 out of 14 patients with antidepressant drug-induced sexual dysfunction (Reference Fava, Rankin and AlpertFava et al, 1998). The benefits of sildenafil may also apply to patients with schizophrenia, as a case report has described successful treatment of reduced sexual desire and erectile failure in a man with schizophrenia who was receiving antipsychotic drugs (Reference Benatov, Reznik and ZemishlanyBenatov et al, 1999). A recent double-blind placebo-controlled study of 160 men with erectile dysfunction and comorbid minor depression found that the response of erectile problems to sildenafil treatment was associated with a significant reduction in depressive symptoms (Reference Seidman, Roose and MenzaSeidman et al, 2001).
Conclusions
The key points of this article are summarised in Box 2. Accurate assessment of sexual function in depression or schizophrenia is subject to many difficulties, including the fact that there are few available data on prevalence of sexual dysfunction in the general population and the effects of antidepressant and antipsychotic drugs. Obtaining accurate data on an intimate subject is potentially prone to underreporting unless careful attention is paid to the method of data collection. Until data from rigorous, controlled, comparative studies are available, the perceived likelihood of sexual side-effects should not unduly influence the choice of psychotropic drug, and prescribing decisions should be made on an assessment of the overall balance of the benefits and risks of treatment.
Box 2 Key points
Sexual dysfunction is common in patients with depression or schizophrenia, but is often not reported to doctors; it may be an unrecognised cause of non-adherence to treatment
Some antidepressant drugs (moclobemide, nefazodone, mirtazapine) are less likely to cause sexual problems than others and may be useful when patients have developed treatment-emergent sexual dysfunction
Certain atypical antipsychotics may cause fewer sexual problems than conventional ones, but whether this is due to a reduced incidence of hyperprolactinaemia remains uncertain
Treatment of established sexual dysfunction in a patient with depression or schizophrenia usually requires the combination of pharmacological and psychological approaches
Multiple choice questions
-
1 The following may be helpful adjuvant treatments for patients with SSRI-induced delayed ejaculation:
-
a buspirone
-
b sildenafil
-
c moclobemide
-
d cyproheptadine
-
e the squeeze technique.
-
-
2 Antipsychotic-induced hyperprolactinaemia can result in the following problems:
-
a unilateral gynaecomastia
-
b infertility
-
c vaginismus
-
d priapism
-
e amenorrhoea.
-
-
3 Brief drug holidays may be a useful approach to SSRI-induced sexual dysfunction when:
-
a the patient is receiving fluoxetine
-
b discontinuation symptoms have proved troublesome
-
c previously abstinent patients wish to resume their opiate habit
-
d the patient is suicidal
-
e the patient is undergoing sertraline treatment.
-
-
4 The problem of sexual dysfunction in patients with depression:
-
a is a fiction created by the pharmaceutical industry
-
b may be an unrecognised cause of treatment non-adherence
-
c is relevant only to patients in intimate relationships
-
d can be elicited by sensitive direct questioning
-
e is usually due to the presence of comorbid physical illness.
-
-
5 Erectile failure in a middle-aged man with chronic schizophrenia and diabetes mellitus, undergoing phenothiazine treatment:
-
a may improve if the patient is switched to quetiapine
-
b can be associated with delusions of control
-
c can be caused by peripheral vascular disease
-
d may be due to depression
-
e can respond to sildenafil treatment.
-
MCQ answers

| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | T | a | T | a | F | a | F | a | T |
| b | T | b | T | b | F | b | T | b | T |
| c | F | c | F | c | F | c | F | c | T |
| d | T | d | F | d | F | d | T | d | T |
| e | F | e | T | e | T | e | F | e | T |





eLetters
No eLetters have been published for this article.